Cancer treatment: Hemerion secures an additional €2 million in funding from Bpifrance
Cancer treatment: Hemerion secures an additional €2 million in funding from Bpifrance
VILLENEUVE D'ASCQ, FRANCE, April 18, 2023/EINPresswire.com/ -- As part of the Deeptech plan, Hemerion obtains a new financing of 2 million € from Bpifrance. This financial aid completes the resources committed by the healthtech start-up for the clinical validation of its technology for the treatment of the most aggressive brain cancer.
Hemerion develops an innovative therapeutic approach based on the combination of a drug (Pentalafen®) and a photonic device (Heliance® Solution).
Improving the treatment of brain cancer
The first indication for this technology focuses on the treatment of glioblastoma, one of the most aggressive brain cancers, which affects approximately 30,000 new patients each year in Europe and the US.
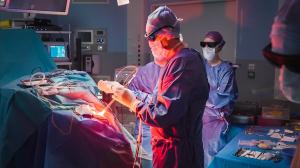
Pentalafen® is administered to the patient before the tumor is removed and concentrates in the tumor cells while being eliminated by healthy cells.
Immediately after tumor resection, the neurosurgeon illuminates the cavity with Heliance® solution. The light activates the Pentalafen® which destroys the remaining cancer cells. The tumor is eradicated wherever the light penetrates.
The results of the Phase I trials conducted in 2017 and 2018 with the Lille University Hospital were published in the Journal of Neuro-Oncology in 2021. They demonstrate the safety and tolerance of the treatment as well as very encouraging initial efficacy results. The treatment could significantly improve survival and quality of life for patients.
A technology that fits perfectly into the standard of care
Hemerion technology fits seamlessly into the standard of care, complementing surgery, radiotherapy and chemotherapy.
Co-developed with a team of neurosurgeons, it naturally takes its place in the operating room, complementing tumor resections.
The treatment developed by Hemerion aims to improve current management of a disease with a very poor prognosis, with a median life expectancy at diagnosis of less than 18 months.
One goal: accelerating the implementation of Phase II clinical trials
Granted as part of Bpifrance’s Deeptech Plan, this financing will enable Hemerion to accelerate the implementation of Phase II clinical trials, currently under discussion with the Food and Drug Administration (FDA) for a first patient inclusion at the end of 2023.
It also opens new perspectives for the company to extend the technology to other indications in the oncology sector.
This aid obtained from Bpifrance consists of two payments via the French government’s Investment Program for the Future: €1.5 million in the form of a recoverable advance, and €0.5 million in the form of grants.
Bpifrance’s Deeptech development aid finances research and development phases of disruptive innovation projects before their industrial and commercial launch. Initiated in 2019, the Deeptech plan is endowed with €3 billion over five years and aims to create 500 start-ups each year.
“This financing complements our initial fundraising efforts very well and paves the way for a more rapid initiation of Phase II clinical trials in glioblastoma with our U.S. clinical partners. It also gives us the means to test our technology in other types of cancers, which would strengthen our pipeline“, said Maximilien Vermandel, CEO of Hemerion.
Next step: securing A series A funding
Bpifrance’s financing puts Hemerion in a very favorable position to raise a Series A round of financing. This step is essential to finance the Phase II and III clinical trials required to commercialize the first Hemerion solution.
“This support from Bpifrance brings our financing to more than €7 million in 2 years. This is a great sign of confidence in our technology and an ideal way to prepare for future fundraising“, says Michel Andraud, Hemerion’s CFO.
Maximilien Vermandel
Hemerion Therapeutics
contact@hemerion.com
Visit us on social media:
Twitter
LinkedIn
YouTube
Hemerion's technology mechanism of action against brain cancer
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.