Hemerion Therapeutics successfully raises over €3.5 million: a new hope for the treatment of brain cancer
This new round of financing accelerates the implementation of international clinical trials aiming at validating Hemerion's solution against glioblastoma.
VILLENEUVE D'ASCQ, HAUTS-DE-FRANCE, FRANCE, April 14, 2022 /EINPresswire.com/ -- Hemerion Therapeutics has successfully completed a €3.55 million seed round combining investment funds, business angels and a loan from Bpifrance. This new round of financing accelerates the implementation of international clinical trials aiming at validating Hemerion's therapeutic solution for the treatment of glioblastoma, the most aggressive brain cancer.
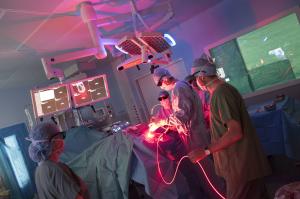
Hemerion's technology combines molecules and innovative laser treatment to selectively destroy cancer cells without damaging healthy tissue. The technology is based on a drug that selectively accumulates in cancer cells. When illuminated by a specific laser light, the drug causes tumor cells death through photochemical cytotoxic effect. Tumor cells are eliminated wherever the light penetrates.
Used as an add-on to surgery, before any adjuvant treatments, Hemerion technology eliminates cancer cells in places that are inaccessible to standard tumor resection. It holds particular promise for the treatment of glioblastoma, one of the most common and aggressive brain tumors. Hemerion Therapeutics has already initiated its clinical program with a first conclusive clinical trial in patients harboring glioblastoma. Its integration into the standard of care could significantly improve patient survival and quality of life.
A high level of seed funding that directly contributes to international clinical development.
With a total amount of €3.55 million, this significant fundraising provides Hemerion's founders and their teams with excellent development prospects.
FIRA NORD EST 2 (Finovam gestion), CapTech Santé Nutrition and Nord France Amorçage funds and several business angels participated in this round with a total investment of €2.55 million. These equity operations are coupled with a €1 million seed loan from Bpifrance.
This new financing follows the first round achieved by Hemerion's founders, the University of Lille Foundation, Bpifrance and a French business angel entrepreneur. Hemerion had then raised a total of more than €1.7M in funding since the company's creation in September 2020.
This new round of funding will notably strengthen the company's regulatory, clinical and industrial resources and prepare for a Series A in 2023.
Accelerated clinical development , particularly in the U.S.
The company is already actively working with the Food and Drugs Administration (FDA) to obtain the Investigational New Drug (IND) label. This step precedes the implementation of a phase II clinical trial in the first half of 2023, in collaboration with Mount Sinai Hospital in New York.
The trial, involving 10 to 15 patients, will validate the safety of the new Heliance technology platform and foreshadow the clinical trial phase: a pivotal step in bringing Hemerion’s solution for the treatment of glioblastoma to market.
Resources for new therapeutic applications in sight
This new funding also allows Hemerion to develop the portfolio of therapeutic applications for its technology. Several preclinical evaluations are already underway to validate the efficacy of the therapeutic solution in the treatment of other types of cancers.
About Hemerion Therapeutics
www.hemerion.com
About FINOVAM Gestion
www.finovamgestion.fr
About CAPTECH Santé Nutrition
www.finorpa.fr
About Nord France Amorçage
www.nord-france-amorcage.fr
Maximilien Vermandel
Hemerion Therapeutics
contact@hemerion.com
Hemerion's technology mechanism of action against brain cancer
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.