Season of support – urgent call for Victorians to sign up for clinical trials
Nucleus Network is highlighting the need for Victorians to take part in clinical trials that can make a big impact around the world.
MELBOURNE, VICTORIA, AUSTRALIA, March 15, 2022 /EINPresswire.com/ -- With Autumn beginning, Australia’s largest Phase 1 clinical trial provider, Nucleus Network, is highlighting the ongoing vital need for Victorians to take part in clinical trials to help evaluate new medications and treatments that can make a big impact to people with health problems around the world.With the lockdowns and border closures of the past two years isolating Victoria, many sectors have been impacted by a lack of international travellers. This includes medical research and clinical trials, which depends on everyday healthy Victorians and travellers, typically 18-55 years, volunteering to participate in studies.
Victorians in particular have stepped up at an unprecedented rate and should be proud of how they’ve helped push forward new and exciting treatments for conditions such as Motor Neurone disease (MND), Alzheimer’s, Malaria, Osteoporosis, Multiple sclerosis (MS), and of course, treatments and vaccines for Covid-19.
Charlotte Hall is the Chief Operating Officer of Nucleus Network, which has Victorian clinics in Melbourne and Geelong. “Before Covid-19 hit, a lot of international travellers and students would put their hand up along-side Victorian locals to join clinical trials of new medications and treatments,” she said.
“During the border closures and lockdowns, and particularly our highly publicised Covid-19 vaccine trial Novovax, Victorians stepped up in their thousands to fill this gap, volunteering to be part of these trials to evaluate new medications. As a result, we’ve been able to deliver on dozens of studies that help us understand potentially life-changing medicines that are being brought closer to being made available to those around the world who need them.”
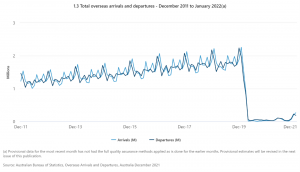
While borders have since reopened, the number of travellers to Australia is still far below the pre-pandemic level. According to the Australian Bureau of Statistics , January 2020 had 2.25 million arrivals, while January 2022 saw the highest number of visitors since then, but still only 260 thousand arrivals, a 90% drop in new arrivals.
“We have had such an enormous response from the people of Victoria,” said Hall. “Not just for trials of Covid-related treatments, but studies related to many other health conditions.”
“In late 2021, we added a Geelong site to our operations, and more than a thousand people have already registered with that clinic to participate in clinical trials. With their participation, Australia has added to our reputation as a world-leader in medical research, pairing the knowledge and capability of our health sector with motivated members of the public that are interested in helping bring the next generation of treatments and medications to those that need them.”
Another participant is Hannah: “While, yes, initially the money was a factor,” she said, “The idea of being able to give my time and the healthy body that I'm pretty fortunate to have, to help somebody else out was actually a really appealing prospect in participating in a study. So for me, it was in the end, more about being able to make a difference in the lives of probably people I'll never meet, just because I've been really fortunate to not have any struggles of my own.”
“We’re incredibly grateful to everyone who has participated so far,” says Hall. “But as always, there are always more trials needing people willing to join them. Every participant is vital, and sometimes it can come down to getting just one more person for a trial to go ahead, one person can really make the difference between a medication rolling out on time or being delayed for years.”
“There’s lots of confusion about what clinical trials are, but our Phase 1 trials are generally looking at how a healthy person metabolises a medication, and that information is used as a baseline for further studies in other phases.”
“There’s a lot of exciting treatments on the horizon. The pandemic highlighted the need to increase medical research and support emerging technologies in the sector, but all of these medications need to be thoroughly evaluated, which is where clinical trials come in. We’re like an independent evaluator, and we also have every trial approved by an independent ethics committee.”
While many trial participants join a study for altruistic reasons, participants are also compensated for their time.
“Participants join a trial for a lot of different reasons,” said Hall. “Some want to help work on treatments for a particular condition that has affected someone they know; others just want to help in any way possible. Participants are also compensated at a rate of $480 per day, which for some longer studies can add up quickly.”
Anyone interested in participating in clinical trials can look at what trials are currently recruiting and register their details at nucleusnetwork.com or call us on 1800 243 733.
About Nucleus Network
Nucleus Network is Australia’s largest Phase 1 clinical research organization and the only Phase 1 specialist globally with facilities in the USA and Australia. Since our establishment in 2004, Nucleus Network has conducted well over 1000 Phase 1 clinical trials for some of the most reputable Biotechnology and Pharmaceutical Companies from around the world.
Our Australian Phase 1 facilities are in Melbourne and Brisbane, with a satellite clinic located in Geelong. Our USA Phase 1 facility is in Minneapolis. Combined, our clinics offer over 200 beds. All three clinics are strategically co-located within leading medical, research and biotech precincts; the Alfred Hospital in Melbourne, the Royal Brisbane and Women’s Hospital in Brisbane, and Medical Alley in Minnesota.
These precincts provide Nucleus Network with unique access to highly specialized ancillary services for the conduct of complex Phase 1 clinical trials. From specialist PD equipment for first in human studies in Melbourne, purpose-built infrastructure for high volume biosimilar studies in Brisbane, and onsite dialysis capabilities for complicated renal studies in Minneapolis, Nucleus Network has the experience to conduct the most complex early phase clinical trials.
Together with our clients, we are fulfilling our purpose of "Advancing medicine, improving lives."
1 https://www.abs.gov.au/statistics/industry/tourism-and-transport/overseas-arrivals-and-departures-australia/dec-2021
Joshua Eddy
Nucleus Network
+61 404 446 174
j.eddy@nucleusnetwork.com.au
Visit us on social media:
Facebook
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.