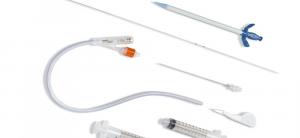
S-Cath™ System for Suprapubic Catheterization adopted by Interventional Radiologists in Texas
ABHI US Accelerator member, Mediplus, announces S-Cath™ use by Texas Interventional Radiologists. PSS Urology is the Exclusive U.S. Distributor for S-Cath™.
SPOKANE VALLEY, WASHINGTON, UNITED STATES, March 30, 2021 /EINPresswire.com/ -- Utilizing the Seldinger Technique, the S-Cath™ System for Suprapubic Catheterization offers several advantages to the clinical staff and the patient including greater control, accuracy and reduced risk of trauma.The S-Cath™ System is latex-free and provides a 40.75% reduction in Catheter Acquired Urinary Tract Infections (CAUTI).
The S-Cath™ System is the standard of care in the UK and is enjoying rapid adoption in the U.S. Market.
When compared to other Suprapubic Catheter Kits, the S-Cath™ System provides a less traumatic approach with similar drainage benefits of larger devices. Clinicians enjoy greater confidence inserting the trocar as the track has already been secured by the guidewire. The safety guidewire improves insertion and removal, guaranteeing accurate placement of the trocar along the anesthetized track.
The procedure rarely needs to be performed as an inpatient, under general anesthetic, or in an operating room, reducing hospital stay and overall cost. Studies show average procedure time of less than 29 minutes.
The S-Cath™ System was developed by Mediplus in close collaboration with Bristol Urology at NHS Southmead Hospital Bristol.
James Urie, sales & marketing director at Mediplus, said: “We are delighted that a British developed device is now being used in Texas to support clinicians stateside. Mediplus is committed to developing safer and easier solutions for urology and we are proud that our device is now being used by interventional radiologists. S-Cath System is available exclusively through PSS Urology.”
The award-winning medical device manufacturer is currently part of the ABHI US Accelerator programme, in partnership with Dell Medical School. Over the last four years, ABHI’s missions to the US have helped UK companies develop and strengthen partnerships across the country with a variety of organizations including academic, investor communities and professional service providers. Outcomes from the missions have included clinical trials, research collaborations, new partnerships and sales.
Paul Benton, managing director, international ABHI, said: “This is a great example of how a UK developed device is now being used by clinicians to improve patient safety. The UK is a leader in innovation, and I am delighted that through programmes like the US Accelerator clinicians are able to access UK technologies and introduce them into standard care to help improve clinical technique and patient outcomes.”
PSS Urology is proud to be the exclusive U.S. distributor of the award winning S-Cath™ System: https://www.s-cath.com
Please contact us for quotations and additional information.
Traves Brady
PSS Urology, Inc.
+1 509-999-3107
email us here
Visit us on social media:
Twitter
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.