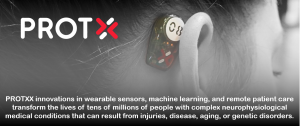
PROTXX phybrata sensing enhances in-clinic and remote patient care for complex neurological conditions
MENLO PARK, CA, UNITED STATES, February 25, 2021 /EINPresswire.com/ -- Silicon Valley, California and Calgary, Alberta based precision medicine technology pioneer PROTXX today announced a powerful new wearable sensing approach to in-clinic and remote patient care and management of complex neurological conditions that can result from injuries, disease, aging, or genetic disorders.
Neuro-disruptive conditions such as stroke and concussion, along with neuro-degenerative conditions such as multiple sclerosis and Parkinson’s disease, can lead to disruptions that are widespread throughout the brain. As a result, patients typically suffer from impairments to multiple physiological systems in their bodies, with highly individual impairment profiles and symptoms that fluctuate significantly. Current solutions for diagnosing these multiple impairments and monitoring the effectiveness of treatments and rehabilitation either (i) require multiple time-consuming tests carried out by multiple clinical specialists using expensive lab equipment, or (ii) are limited to subjective observations and reliance on patient self-reporting. These limitations lead to sub-optimal patient quality of care and outcomes, along with billions of dollars in healthcare cost inefficiencies.
The PROTXX physiological vibration acceleration (“phybrata”) sensor delivers an innovative solution to the above problem by enabling much easier to use, lower cost, in-clinic and remote patient assessments, as described in a landmark study recently published in the journal Medical Devices: Evidence and Research and entitled “Physiological Vibration Acceleration (Phybrata) Sensor Assessment of Multi-System Physiological Impairments and Sensory Reweighting Following Concussion“. The paper is available for download at https://www.dovepress.com/getfile.php?fileID=64560.
Ashutosh Raina, MD, pediatric neurologist at the Center of Excellence for Pediatric Neurology in Sacramento and Rocklin, California, co-founder of the Concussion Medical Clinic in Rocklin, California, and a co-author on the study commented “The PROTXX phybrata solution provides important new capabilities for personalizing care and rehabilitation of people with complex neurophysiological conditions, allowing us to improve patient quality of care while saving significant healthcare dollars.”
Brian Benson, MD, Chief Medical Officer at the Benson Concussion Institute in Calgary, Alberta and also a co-author on the study added “Phybrata testing provides us with an important adjunct to standard concussion assessment tools by objectively ascertaining neurological and vestibular impairments, guiding targeted rehabilitation strategies, monitoring recovery, and assisting with return-to-sport/work/learn decision-making.”
The phybrata sensor attaches behind the ear using a small disposable adhesive and detects microscopic involuntary motions of the body, both normal and pathological. The PROTXX machine learning engine leverages unique features in the phybrata signals to identify and analyze the different contributions made by each of the body’s physiological systems to a phenomenon that is unique to a head-mounted sensor, the stabilization of the head and eyes as the reference platform used by the body to enable balance and movement. This unique sensing capability allows a one-minute, non-invasive phybrata test to identify, quantify, and monitor impairments using the unique biomechanical vibrational signature of each physiological system. Results are presented in a simple, intuitive test summary for clinicians and patients.
John Ralston, PhD, PROTXX CEO and founder further described how “COVID-19 has accelerated demand for remote patient monitoring applications of the PROTXX phybrata platform, along with new capabilities that we are launching in 2021, including comprehensive phybrata gait analysis and extended monitoring of daily activities. Our expanding healthcare provider customer base, collaborations with leading academic and clinical research teams, and partnerships with international medtech distributors all validate the size of the global opportunity that all of these phybrata capabilities address.”
James Hayward, Principal Technology Analyst covering wearable technologies at IDTechEx, followed up on the recent PROTXX phybrata product announcement: “PROTXX innovations in wearable devices, machine learning, and remote patient care solve the difficult problem of managing the many different physiological impairments that can accompany complex neurological medical conditions. By disrupting diagnosis and treatment with easy-to-use, low-cost, precision patient assessments, PROTXX has set a new standard for clinical applications of wearable technologies.”
******
About PROTXX, Inc. (https://protxx.com/)
PROTXX innovations in wearable sensors, machine learning, and remote patient care transform the lives of tens of millions of people with complex neurophysiological medical conditions that can result from injuries, disease, aging, or genetic disorders. Headquartered in Silicon Valley, with Canadian operations based in Calgary, PROTXX is led by an accomplished team of IoT device and data platform engineers, clinical neurology researchers and practitioners, and digital healthcare business professionals. Supported by a well-established network of R&D, manufacturing, clinical pilot, and distributor partners in Canada and the U.S., PROTXX has been recognized with numerous industrial, academic, and government awards.

Neuro-disruptive conditions such as stroke and concussion, along with neuro-degenerative conditions such as multiple sclerosis and Parkinson’s disease, can lead to disruptions that are widespread throughout the brain. As a result, patients typically suffer from impairments to multiple physiological systems in their bodies, with highly individual impairment profiles and symptoms that fluctuate significantly. Current solutions for diagnosing these multiple impairments and monitoring the effectiveness of treatments and rehabilitation either (i) require multiple time-consuming tests carried out by multiple clinical specialists using expensive lab equipment, or (ii) are limited to subjective observations and reliance on patient self-reporting. These limitations lead to sub-optimal patient quality of care and outcomes, along with billions of dollars in healthcare cost inefficiencies.
The PROTXX physiological vibration acceleration (“phybrata”) sensor delivers an innovative solution to the above problem by enabling much easier to use, lower cost, in-clinic and remote patient assessments, as described in a landmark study recently published in the journal Medical Devices: Evidence and Research and entitled “Physiological Vibration Acceleration (Phybrata) Sensor Assessment of Multi-System Physiological Impairments and Sensory Reweighting Following Concussion“. The paper is available for download at https://www.dovepress.com/getfile.php?fileID=64560.
Ashutosh Raina, MD, pediatric neurologist at the Center of Excellence for Pediatric Neurology in Sacramento and Rocklin, California, co-founder of the Concussion Medical Clinic in Rocklin, California, and a co-author on the study commented “The PROTXX phybrata solution provides important new capabilities for personalizing care and rehabilitation of people with complex neurophysiological conditions, allowing us to improve patient quality of care while saving significant healthcare dollars.”
Brian Benson, MD, Chief Medical Officer at the Benson Concussion Institute in Calgary, Alberta and also a co-author on the study added “Phybrata testing provides us with an important adjunct to standard concussion assessment tools by objectively ascertaining neurological and vestibular impairments, guiding targeted rehabilitation strategies, monitoring recovery, and assisting with return-to-sport/work/learn decision-making.”
The phybrata sensor attaches behind the ear using a small disposable adhesive and detects microscopic involuntary motions of the body, both normal and pathological. The PROTXX machine learning engine leverages unique features in the phybrata signals to identify and analyze the different contributions made by each of the body’s physiological systems to a phenomenon that is unique to a head-mounted sensor, the stabilization of the head and eyes as the reference platform used by the body to enable balance and movement. This unique sensing capability allows a one-minute, non-invasive phybrata test to identify, quantify, and monitor impairments using the unique biomechanical vibrational signature of each physiological system. Results are presented in a simple, intuitive test summary for clinicians and patients.
John Ralston, PhD, PROTXX CEO and founder further described how “COVID-19 has accelerated demand for remote patient monitoring applications of the PROTXX phybrata platform, along with new capabilities that we are launching in 2021, including comprehensive phybrata gait analysis and extended monitoring of daily activities. Our expanding healthcare provider customer base, collaborations with leading academic and clinical research teams, and partnerships with international medtech distributors all validate the size of the global opportunity that all of these phybrata capabilities address.”
James Hayward, Principal Technology Analyst covering wearable technologies at IDTechEx, followed up on the recent PROTXX phybrata product announcement: “PROTXX innovations in wearable devices, machine learning, and remote patient care solve the difficult problem of managing the many different physiological impairments that can accompany complex neurological medical conditions. By disrupting diagnosis and treatment with easy-to-use, low-cost, precision patient assessments, PROTXX has set a new standard for clinical applications of wearable technologies.”
******
About PROTXX, Inc. (https://protxx.com/)
PROTXX innovations in wearable sensors, machine learning, and remote patient care transform the lives of tens of millions of people with complex neurophysiological medical conditions that can result from injuries, disease, aging, or genetic disorders. Headquartered in Silicon Valley, with Canadian operations based in Calgary, PROTXX is led by an accomplished team of IoT device and data platform engineers, clinical neurology researchers and practitioners, and digital healthcare business professionals. Supported by a well-established network of R&D, manufacturing, clinical pilot, and distributor partners in Canada and the U.S., PROTXX has been recognized with numerous industrial, academic, and government awards.
John Ralston
PROTXX Inc
john.ralston@protxx.com
Visit us on social media:
LinkedIn
Introducing the PROTXX "phybrata" platform
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.