New Clinical Study Shows Promise for Dietary Management of Hyperoxaluria
Innovative enzyme was effective in 94% of study subjects
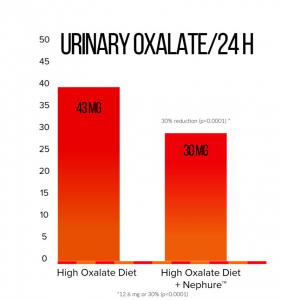
Ox-1, Captozyme’s proprietary oxalate decarboxylase enzyme — marketed as Nephure™ and distributed through their subsidiary, Entring LLC — significantly decreased urinary oxalate in 94% of participants, as demonstrated by the prospective, double-blind, randomized, placebo-controlled, cross-over study of 33 healthy volunteers. Within-subject mean difference in 24-hour urinary oxalate was 12.6 mg or 30% (p<0.0001) when comparing treatment vs. baseline on a high-oxalate diet.
“Compliance with physician’s recommendations is imperative, but following dietary recommendations can be challenging for many individuals. A product that can help with compliance will offer value to everyone involved. We are delighted to share these results with the medical community, confirming the efficacy and potency of our oxalate-reducing enzyme to reduce urinary oxalate,” Helena Cowley, Chief Executive Officer of Captozyme Inc., said.
The clinical study, sponsored by Captozyme Inc., began in August 2018. Following screening and baseline evaluations, all study subjects were placed on a two-day high-oxalate diet lead-in that resulted in an elevation of urinary oxalate to approximately 43mg/24 hours. Subjects were then randomized to one of two cross-over treatment sequences (Nephure™ to placebo or placebo to Nephure™) while maintaining the controlled high-oxalate, low-calcium meal plan consisting of standard Western meals with added spinach and rhubarb to provide additional levels of oxalate intake. Study subjects ate between 750 to 800 mg of oxalate and 500 to 550 mg of calcium per day and took Nephure™ or placebo three times per day for two days. At the end of the first treatment period, subjects experienced a two-day wash-out, after which they crossed over and were administered the alternate treatment.
Eight 24-hour urine collections were performed during the entirety of the study. Apart from the reduction of urinary oxalate, researchers found no other significant differences in any of the urinary parameters measured in the study, including calcium, magnesium, citrate, uric acid and creatinine.
“We attribute the high potency and significant reduction — despite a low dose of enzyme — to the high affinity our enzyme has to oxalate, as well as its sustained activity in the low pH of the stomach. We are excited to continue our clinical development program and further evaluate the potential of this enzyme at higher dose levels,” Helena Cowley remarked.
The clinical study will be published later this year. For more information about the clinical study or to download a summary, visit Captozyme.com/ClinicalStudy.
About Captozyme
Captozyme, Inc. is a biotechnology company developing enzymes and formulations to advance knowledge in the area of gut health and through its products, advance the health and wellness of people. Captozyme, Inc. also houses a cGMP manufacturing facility for production of Phase 1 clinical trial drug substance with a focus on live biologics development. The company is based in Gainesville, Florida. Learn more about Captozyme by visiting www.captozyme.com.
###
Amanda Austin
Captozyme Inc.
+1 352-558-8845
email us here
Visit us on social media:
Facebook
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.