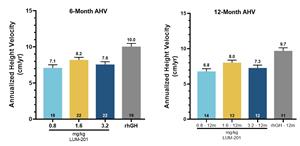
Iovance Biotherapeutics Reports Third Quarter and Year-to-Date 2023 Financial Results and Corporate Updates
FDA Priority Review of Biologics License Application (BLA) on Track for Lifileucel in Advanced Melanoma with Prescription Drug User Fee Act Action (PDUFA) Date of February 24, 2023 Positive Regulatory Feedback Supports Lifileucel Regulatory Submissions in …