FDA Expands 510(k) Clearance for MERIT’s Cloud-Based Imaging Management Platform, EXCELSIOR
This expansion of our 510(k) clearance shows our commitment to developing new capabilities for EXCELSIOR while maintaining the highest regulatory standards.”
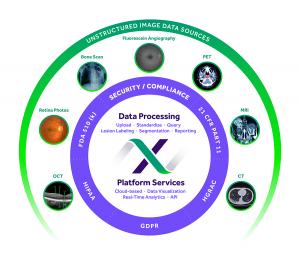
MADISON, WI, UNITED STATES , November 15, 2022 /EINPresswire.com/ -- MERIT CRO, Inc., a global endpoint service provider specializing in multiple therapeutic areas, announced today that its EXCELSIOR(TM) imaging software has received expansion of its FDA 510(k) market clearance (K#220929). The expanded clearance includes new indications for use such as interpretation and evaluation of radiologic images (CT, MR and PET) in clinical trials. In 2013, EXCELSIOR received FDA 510(k) clearance as a class 2 medical device for managing ophthalmic clinical trial data.— Nathan Diers, VP of Product Development at MERIT
“We are excited to bring EXCELSIOR’s latest imaging technologies for data standardization, analysis, and reading to new indications,” said Yijun Huang, Co-Founder and CEO of MERIT. “This new expansion furthers our efforts to be ‘your clinical trial endpoint expert.’”
Nathan Diers, VP of Product Development at MERIT, said “EXCELSIOR is a cloud-based software platform that offers end-to-end workflow management for managing the clinical data and images in clinical trials. This expansion of our 510(k) clearance shows our commitment to developing new capabilities for EXCELSIOR while maintaining the highest regulatory standards.”
ABOUT MERIT
MERIT (https://meritcro.com/) is an innovative, global clinical trial endpoint services provider working in a variety of therapeutic areas, including ophthalmology, respiratory, oncology, cardiac safety, dermatology, and neurology. We partner with CROs as well as pharmaceutical and biotech companies to deliver reliable endpoint services in multi-regional clinical trials. Together our work advances and accelerates the improvement of therapeutic options for patients worldwide.
MERIT’s EXCELSIOR technology platform increases accuracy and efficiency by providing a suite of advanced endpoint analysis tools designed based on our extensive collaboration with biopharma companies.
Stacy Sanderson
MERIT CRO
+1 608-284-8810
email us here
Visit us on social media:
Twitter
LinkedIn
Other
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.