BioRegenx Announces Microvascular Differences Study in People with Obesity at Risk of Developing Cardiovascular Disease
The paper is titled Microvascular Differences in Individuals With Obesity At Risk of Developing Cardiovascular Disease. The senior author is Dr. Hans Vink, the inventor of GlycoCheck™ and Chief Science Officer of BioRegenx. The objective was to investigate microvascular differences in individuals with obesity at risk for developing cardiovascular disease.
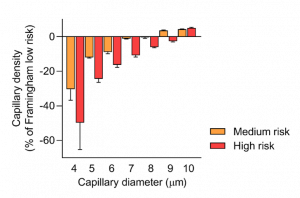
GlycoCheck™ shows that increased cardiovascular risk reduces capillary density by 49% in healthy individuals in this obesity study, as shown in this chart.
Obesity is a well-established risk factor for developing cardiovascular disease (CVD), the leading cause of mortality worldwide. Because microvascular dysfunction is one of the first signs of CVD, the study’s researchers aimed to investigate whether individuals with obesity and a high risk for developing CVD could be characterized by microvascular changes measured with sidestream dark field (SDF) imaging.
The Framingham Risk Score was used to calculate 10-year cardiovascular risk, divided into low-, intermediate-, and high-risk groups. The Framingham Risk Score is a sex-specific algorithm that is widely used to assess the risk of cardiovascular events (coronary, cerebrovascular, and peripheral artery disease and heart failure) within 10 years.
Study Importance
What is already known?
►Microvascular changes due to endothelial dysfunction are an early step in the pathogenesis of cardiovascular disease. Sidestream dark field imaging is a noninvasive technique to detect these microvascular changes.
What does this study add?
►By analyzing densities of blood perfused microvessels in a diameter-dependent manner, it is demonstrated that significant reductions in the number of the smallest capillaries can be identified in individuals with obesity exposed to increased cardiovascular risk according to the Framingham Risk Score. This new analysis could be used for early detection of microvascular changes in individuals with obesity that might aid in monitoring such individuals, e.g., using various interventions.
How might your results change the direction of research or the focus of clinical practice?
►By adding red blood cell velocity calculations to the new sidestream dark field imaging software, a better estimate of perfused boundary regions can be implemented that can be used to detect early signs of microvascular damage. Automated imaging can be used to detect early microvascular changes and monitor clinical interventions aimed at restoring microvascular health.
Results
A total of 813 participants were included. The high-risk group (n = 168) was characterized by differences in the microvasculature compared with the low-risk group (n = 392): the high-risk group had a 49% reduction in the number of smallest capillaries and a 9.1-µm/s (95% CI: 5.2-12.9) higher red blood cell velocity in the feed vessels. No differences in velocity-corrected perfused boundary regions were found.
This study was supported by a grant (LSHM16058-SGF) for the GLYCOTREAT consortium, a collaboration project financed by the public-private partnership (PPP) allowance made available by Top Sector Life Sciences & Health to the Dutch Kidney Foundation to stimulate public-private partnerships. The Netherlands Epidemiology of Obesity (NEO) study is supported by participating departments, the Division and the Board of Directors of the Leiden University Medical Center, and by the Leiden University Research Profile Area “Vascular and Regenerative Medicine.” The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
The study was conducted by the Division of Nephrology, Department of Internal Medicine, The Einthoven Laboratory for Vascular and Regenerative Medicine, Leiden University Medical Center, Leiden, the Netherlands; Department of Clinical Epidemiology, Leiden University Medical Center, Leiden, The Netherlands; Department of Nephrology, Nijmegen Centre for Molecular Life Sciences, Radboud University Medical Centre, Nijmegen, The Netherlands; Department of Cardiology, Leiden University Medical Center, Leiden, The Netherlands; and the Department of Physiology, Cardiovascular Research Institute Maastricht, Maastricht, The Netherlands.
About GlycoCheck
GlycoCheck™, an FDA registered Class 1 medical testing device developed by GlycoCheck B.V., analyzes glycocalyx, endothelium, and capillary function. It analyzes capillaries that are as small as 4 microns, so small that 100 of these tiny capillaries fit inside a human hair. GlycoCheck™ testing has been used as part of 92 peer-reviewed research studies from hospitals and universities worldwide. GlycoCheck is patented in the U.S., Canada, Europe, China, and Japan and is exclusively distributed worldwide by MVHS.
About BioRegenx
BioRegenx, Inc., (BioRegenx.com) is a holding company with four subsidiaries, Microvascular Health Solutions, LLC, MyBodyRx, LLC, NuLife Sciences, Inc., and Regenr8, Inc. BioRegenx was created to integrate leading-edge companies into one synergistic platform offering 360-degree solutions, which include leading-edge testing technologies and nutraceutical solutions. Testing technologies include the breakthrough GlycoCheck™, developed by GlycoCheck, B.V. and exclusively distributed by Microvascular Health Solutions, and TruEpigentics DNA and epigenetic testing. Nutraceuticals include the patented Endocalyx Pro™ and additional synergistic dietary supplements sold under the MyBodyRx brand.
Safe Harbor
This press release contains forward-looking information within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended (the "Exchange Act"), including statements regarding potential sales, the success of the company's business, as well as statements that include the word believe or similar expressions. Such forward-looking statements involve known and unknown risks, uncertainties, and other factors that may cause the actual results, performance, or achievements of BioRegenx, Inc. to differ materially from those implied or expressed by such forward-looking statements. This press release speaks as of the date first set forth above, and BioRegenx, Inc. assumes no responsibility to update the information included herein for events occurring after the date hereof. Actual results could differ materially from those anticipated due to factors such as the lack of capital, timely development of products, inability to deliver products when ordered, inability of potential customers to pay for ordered products, and political and economic risks inherent in international trade.
William Resides
BioRegenx
+1 800-398-9842
email us here
Visit us on social media:
Facebook
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.