Progressive Supranuclear Palsy (PSP) Therapies Market, 2021-2030
Progressive Supranuclear Palsy (PSP) Therapies Market, 2021-2030
LONDON, ENGLAND, UNITED KINGDOM, October 25, 2021 /EINPresswire.com/ -- To order this 100+ page report, which features 70+ figures, please visit https://www.rootsanalysis.com/reports/progressive-supranuclear-palsy-market.html%20
Roots Analysis has announced the addition of “Progressive Supranuclear Palsy Therapies Market, 2021-2030” report to its list of offerings.
Key Inclusions
A detailed assessment of the therapeutic pipeline activity and therapeutic assessment of the products by development stage, type of molecule, mechanism of action, type of treatment, orphan drug designation, route of administration, other target disease indications, as well as information on developer’s location, employee base, ownership and year of establishment.
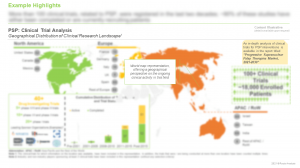
An in-depth analysis of completed, and ongoing studies of various PSP interventions, highlighting various parameters, such as trial registration year, trial phase, number of patients enrolled, study design, trial recruitment status, and trial focus, highlighting leading sponsors, type of organization, regional distribution of trials and type of PSP treatment.
A detailed analysis identifying the key opinion leaders (KOLs), featuring a 2X2 analysis to assess the relative experience of certain KOLs who were shortlisted based on their contributions (in terms of involvement in various clinical studies) to this field.
A detailed study of grants that have been awarded to various research institutes for PSP projects, in the period between 2010-2021 (till July), highlighting analyses based on multiple parameters, such as year of award, amount awarded, administering institute center, support period, type of grant application, purpose of grant award, activity code, emerging focus areas of the grants, popular NIH departments, and type of recipient organization, while highlighting popular recipient organizations, popular program officers and regional distribution of recipient organizations.
An in-depth analysis of the published articles related to PSP and potential therapeutics based on the year of publication, top authors, key journals and emerging focus areas.
An analysis of the partnerships that have been inked by stakeholders in this domain since 2015, covering instances of research and development, licensing, product development, clinical trials, and other relevant types of deals.
Detailed profiles of the players that are engaged in the development of drug products / therapies for progressive supranuclear palsy, featuring overview of the company, its financial information (if available), a description of its product portfolio, recent collaborations and an informed future outlook.
An insightful market assessment summary, highlighting the clinical and commercial attractiveness of pipeline molecules (phase II), taking into consideration target patient population, expected launch date, competitive landscape, annual treatment cost and likely adoption of the therapy.
The report also features the likely distribution of the current and forecasted opportunity across important market segments, mentioned below:
Phase II Drugs
AZP2006
RT001
BRAVYL
Emeramide
NORTHERA
Type of Therapy
Curative
Symptomatic / Palliative
Regional Distribution
North America (US)
Europe (France, Germany, Italy, Spain, UK)
The report also features detailed transcripts of discussions (in reverse chronological order) held with the following experts:
Daniel Brennan (Business and Operations Advisor, NeuroTau)
Fabrizio Stocchi (Director of the Parkinson’s Disease and Movement Disorders Research Center, IRCCS San Raffaele)
To request sample pages, please visit https://www.rootsanalysis.com/reports/progressive-supranuclear-palsy-market/request-sample.html
Key Questions Answered
What are the prevalent R&D trends related to PSP?
What are the key challenges faced by stakeholders developing PSP therapies?
Which are the principal therapies being developed for PSP?
Who are the leading industry and non-industry players in the PSP therapies market?
Which are the key geographies where research on PSP is being conducted?
Which are the leading administering institute centers supporting the research related to PSP therapies?
Who are the key opinion leaders / experts that have contributed to the domain of PSP therapies?
What kind of partnership models are commonly adopted by stakeholders engaged in this industry?
What are the factors that are likely to influence the evolution of PSP therapies market?
How is the current and future market opportunity likely to be distributed across key market segments?
To request a sample copy / brochure of this report, please visit https://www.rootsanalysis.com/reports/progressive-supranuclear-palsy-market.html
You may also be interested in the following titles:
1) Brain Concussion Market, 2021-2031
2) Hunter Syndrome: Pipeline Review, Developer Landscape and Competitive Insights, 2021-2031
3) Pediatric Brain Tumors: Pipeline Review, Developer Landscape and Competitive Insights, 2021-2031
4) Neurotrophic Keratitis: Pipeline Review, Developer Landscape and Competitive Insights, 2021-2031
5) Neuromyelitis Optica Spectrum Disorder (2nd Edition): Pipeline Review, Developer Landscape and Competitive Insights, 2021-2031
Contact:
Ben Johnson
+1 (415) 800 3415
ben.johnson@rootsanalysis.com
Gaurav Chaudhary
Roots Analysis
+919041514099 ext.
email us here
Visit us on social media:
Facebook
Twitter
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.