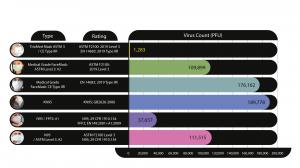
Does Your Mask/Respirator Filter Live Viruses?
MIRABEL, QUEBEC, CANADA, March 25, 2021 /EINPresswire.com/ -- i3 Biomedical inc., a medical device corporation, releases today the comparative results of Live viruses Filtration performances of numerous Medical Face masks and Respirators in the tables included. For a comprehensive understanding, the results are rendered in the form of number of viruses inhaled per Minute per Mask/Respirator based on the average volume of air of 7 liter per minute required by humans. The scientific data is the result of advanced testing executed by GAP EnviroMicrobial Services Ltd., an independent Canadian ISO certified laboratory.
All Procedure masks, Medical Masks and Respirators (further referred to as “Masks”) following the European and/or American regulatory standards are not required to be tested against live viruses, but only against either a solid particle (example: salt particles) or against bacteria (which are approximately 100 times bigger in size than viruses).
The only regulated standards currently used in the Americas and in Europe that include testing the assessment of the filtration performances of microbes are respectively the North American ASTM 2100 and the European EN14683 Bacterial Filtration Efficiency (BFE). While it is not a government requirement, the substitution of the Bacteria for a Live Virus to test Viral Filtration Efficiency (VFE) is accepted. These standardized testing protocols require a concentration of 10^5 viruses per liter of air for only a 2-minute testing time, hence providing the filtration performance of “Masks” in use for 2 minutes only. GAP EnviroMicrobial Services Ltd. was mandated to test the (VFE) following the same protocols but for a testing time of 30 minutes at the NIOSH1 recommended air speed of 85 LPM, on various masks (Procedure masks, Surgical/Medical masks ASTM Level 3, CE Type IIR, KN 95 masks, N95 masks and FFP2 masks). The above-mentioned testing is done on the filter materials only of the masks as per protocol requirements.
For reference, a single cough from an infected subject can release an average of 4,914,600 aerosolized viral particles2. Find the answer to: Does your mask/respirator filter live Viruses in the Tables included.
For more information on the TrioMed Active face masks, visit www.triomed.com or send an email to triomed@triomed.com
About i3 BioMedical Inc: i3 BioMedical is a Canadian medical device corporation, focused on the development and manufacturing of novel antimicrobial products incorporating the TrioMed Active Technology.
About GAP EnviroMicrobial Services Ltd.: GAP EnviroMicrobial Services Ltd. (GAP), established in 1996, is ISO/IEC 17025:2005 compliant with accreditation by the Canadian Association for Laboratory Accreditation (CALA), CALA accreditation is recognized by almost 60 accrediting bodies in 40 countries around the world.
1 National Institute for Occupational Safety and Health
2 Lee, J., Yoo, D., Ryu, S., Ham, S., Lee, K., Yeo, M., Min, K. and Yoon, C. (2019). Quantity, Size Distribution, and Characteristics of Cough-generated Aerosol Produced by Patients with an Upper Respiratory Tract Infection. Aerosol Air Qual. Res. 19: 840-853. https://doi.org/10.4209/aaqr.2018.01.0031
All Procedure masks, Medical Masks and Respirators (further referred to as “Masks”) following the European and/or American regulatory standards are not required to be tested against live viruses, but only against either a solid particle (example: salt particles) or against bacteria (which are approximately 100 times bigger in size than viruses).
The only regulated standards currently used in the Americas and in Europe that include testing the assessment of the filtration performances of microbes are respectively the North American ASTM 2100 and the European EN14683 Bacterial Filtration Efficiency (BFE). While it is not a government requirement, the substitution of the Bacteria for a Live Virus to test Viral Filtration Efficiency (VFE) is accepted. These standardized testing protocols require a concentration of 10^5 viruses per liter of air for only a 2-minute testing time, hence providing the filtration performance of “Masks” in use for 2 minutes only. GAP EnviroMicrobial Services Ltd. was mandated to test the (VFE) following the same protocols but for a testing time of 30 minutes at the NIOSH1 recommended air speed of 85 LPM, on various masks (Procedure masks, Surgical/Medical masks ASTM Level 3, CE Type IIR, KN 95 masks, N95 masks and FFP2 masks). The above-mentioned testing is done on the filter materials only of the masks as per protocol requirements.
For reference, a single cough from an infected subject can release an average of 4,914,600 aerosolized viral particles2. Find the answer to: Does your mask/respirator filter live Viruses in the Tables included.
For more information on the TrioMed Active face masks, visit www.triomed.com or send an email to triomed@triomed.com
About i3 BioMedical Inc: i3 BioMedical is a Canadian medical device corporation, focused on the development and manufacturing of novel antimicrobial products incorporating the TrioMed Active Technology.
About GAP EnviroMicrobial Services Ltd.: GAP EnviroMicrobial Services Ltd. (GAP), established in 1996, is ISO/IEC 17025:2005 compliant with accreditation by the Canadian Association for Laboratory Accreditation (CALA), CALA accreditation is recognized by almost 60 accrediting bodies in 40 countries around the world.
1 National Institute for Occupational Safety and Health
2 Lee, J., Yoo, D., Ryu, S., Ham, S., Lee, K., Yeo, M., Min, K. and Yoon, C. (2019). Quantity, Size Distribution, and Characteristics of Cough-generated Aerosol Produced by Patients with an Upper Respiratory Tract Infection. Aerosol Air Qual. Res. 19: 840-853. https://doi.org/10.4209/aaqr.2018.01.0031
Pierre Jean Messier
i3 Biomedical Inc.
triomed@triomed.com
Visit us on social media:
Facebook
Twitter
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.