ZandCell Announces Availability of Coronavirus Rapid Point-Of-Care Test Kit
Biotech company ZandCell ships COVID-19 rapid response test kit to governments, cities, hospitals, clinics & doctors to administer testing for the virus
ZandCell, a biotechnology company pioneering the advancements and therapeutic applications of Stem Cell Therapy, today announces the COVID-19 Rapid Point-of-Care (POC) Test Kit is now available and shipping worldwide to governments, hospitals, clinics, and doctors to quickly and effectively test patients for the infectious virus and provide immediate results within 10 minutes.
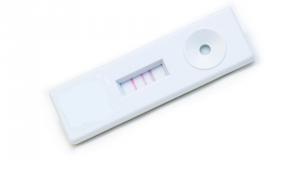
With 99 percent accuracy, the COVID-19 Rapid POC Test Kit rapidly tests to qualitatively detect severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Using the COVID-19 Rapid POC Test Kit, more individuals who are unaware they have been infected can be identified. COVID-19 is determined through a lateral flow test combined with fingerstick blood to reveal the results in 10 minutes.
“Health providers in the U.S. and across the globe have struggled to contain the rapid spread of COVID-19 in part because of a significant shortage of available tests that can quickly, efficiently and cost-effectively provide immediate feedback results,” said Michael Zand, ZandCell CEO. “We have already shipped this test kit to thousands of doctors worldwide and will continue to work around the clock to deliver our products and fight against the virus.”
COVID-19 Rapid POC Test Kit is available to order today in Europe, Asia, Africa, the Middle East and Australia at: www.EasyCoronaTest.com for $49. The kit will be available in the United States following FDA approval.
COVID-19 Rapid POC Test Kit Instructions for use:
Please read the kit instructions carefully before use
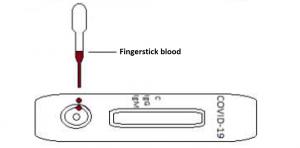
1. Open the test sample and unpack the aluminum foil bag, remove the test cassette and place it on a horizontal table
2. Add a drop of blood to the sample well of the test cassette
3. Immediately add 2 drops of sample dilution solution to the sample well
4. Incubate for 10 – 15 minutes to achieve results (note – reading beyond 30 minutes is invalid)
COVID-19 Rapid POC Test Kit results:
1. Negative: one line C1 (control line)
2. Positive: Two or three (control+one or two extra) strong lines
About ZandCell
ZandCell is a privately held biotechnology company committed to bringing to market life-transforming therapeutics for patients with untreatable diseases. The company focuses on diseases for which the unmet medical need is high, the biology for treatment is clear, and for which there are no current effective treatment modalities.
The company is led by a management team experienced in the development and commercialization of disease therapeutics. ZandCell's strategy is predicated upon time and cost-efficient drug development, with the goal of delivering safe and effective therapies to patients with the utmost urgency. The overall objective of ZandCell and its operations is to develop medical treatments in the fields of regenerative medicine, gene editing, and immunotherapy worldwide. Please visit: https://zandcell.com/ for more information.
*Disclaimer These kits are only available for sale to companies in the healthcare industry, for usage in POC (point of care), and orders can only be placed by medical professionals.
Evan Sneider
Red Rooster PR
+1 954-673-6835
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.