Microenvironment-feedback Hydrogel Enables Precise Staged Repair of Infected Wounds
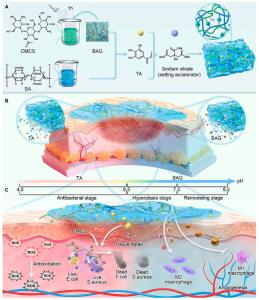
GA, UNITED STATES, December 8, 2025 /EINPresswire.com/ -- A research team from Fudan University has developed a hydrogel technology based on microenvironment-responsive mechanisms. The material can sense pH changes in the wound environment and dynamically release functional agents, enabling a switch from antibacterial action to tissue repair. Constructed from an interpenetrating network of sodium alginate and carboxymethyl chitosan, and loaded with tannic acid and zinc-doped bioactive glass, the hydrogel rapidly releases antibacterial molecules during infection and gradually delivers regenerative ions during healing—achieving, for the first time, precise, stage-specific control of infected wound treatment.
Smart materials are entering the wound care field — and they're learning to respond like doctors. A research team at Fudan University, led by Prof. Xiangchao Meng, has developed a hydrogel that can sense changes in wound pH and automatically switches its therapeutic behavior from fighting infection to promoting tissue repair.
The hydrogel is made from sodium alginate and carboxymethyl chitosan, forming an interpenetrating network that encapsulates two key bioactive components: tannic acid, a natural antibacterial agent, and zinc-doped bioactive glass, which releases ions known to support healing. “In an acidic wound environment, which is typical during infection, the gel contracts and releases tannic acid to kill bacteria and reduce oxidative stress,” explains Meng. “As healing progresses and the pH becomes more alkaline, the gel expands and gradually releases zinc and calcium ions that promote angiogenesis and tissue regeneration.”
The research team aimed to design a material that does not just cover the wound, but also understands what is happening and responds in real-time. “This dual-function system adapts to each healing stage and actively assists the process,” says Meng. “In preclinical rat models with infected wounds, the hydrogel achieved over 90% wound closure in just 14 days, significantly outperforming standard treatments.”
Histological analysis revealed enhanced collagen deposition, reduced inflammation, and improved blood vessel formation. Notably, the gel remains inert in healthy tissue and activates only under pathological conditions, reducing drug overuse and limiting the need for frequent dressing changes. This feature makes it especially promising for treating complex wounds like diabetic foot ulcers or post-surgical infections.
The team is now exploring clinical translation and broader applications. “This is a step toward intelligent wound management,” adds Meng. “Materials that can listen to the body and respond accordingly could redefine how we treat injury and disease.”
Original Source URL
https://doi.org/10.1016/j.bmt.2025.100120
Funding information
This work was supported in part by the Youth Program of Minhang Hospital(2023MHPY01), Shanghai Minhang District Medical Specialty Construction Project(2025MWTZA04), Natural science research projects of Minhang District(2025MHZ015), and Zhejiang Provincial Medicine and Health Technology Project (2025710477).
Lucy Wang
BioDesign Research
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.