NASH Or (MASH) Treatment Market to hit USD$ 31.76 billion by 2033, (CAGR of 17.7%)
NASH (MASH) Treatment Market Growth | Pharma Innovation, NCE Approvals & Revenue Outlook 2032
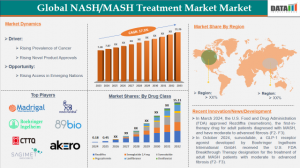
According to DataM Intelligence, the Global NASH/MASH Treatment Market was valued at USD 7.87 billion in 2024 and is projected to expand to USD$ 31.76 billion by 2033, reflecting a CAGR of 17.7% during 2025–2033.
Within this, the approved drugs segment accounted for USD 178.31 million in 2024 and is forecast to surge to USD 16.82 billion by 2033, registering an exceptional CAGR of 57.05%, driven by rapid regulatory approvals and commercial adoption of newly launched therapeutics.
The market growth is driven by the rising global burden of metabolic disorders including obesity, type 2 diabetes, and dyslipidemia leading to an increasing prevalence of non-alcoholic steatohepatitis (NASH), also termed metabolic dysfunction–associated steatohepatitis (MASH).
Rapid advancements in GLP-1 receptor agonists, FXR agonists, FGF21 analogs, and combination therapies have accelerated treatment adoption as pharmaceutical companies race to launch disease-modifying therapies for NASH.
The shift toward non-invasive diagnostics, imaging biomarkers, and AI-enabled liver fibrosis scoring significantly enhances early detection and treatment responsiveness, reducing the need for traditional biopsy.
Get a Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):– https://www.datamintelligence.com/download-sample/nash-or-mash-treatment-market
Growth Drivers
• Global NASH/MASH population reached 47 million patients in 2024, projected to exceed 78 million by 2032.
• Over 70% of NASH patients are obese or diabetic, escalating clinical demand for first-line therapeutics.
• Annual economic burden surpassed USD 120 billion in the U.S. alone in 2024, pressuring early intervention and drug adoption.
• Diagnostic & screening rates increased 2.2× between 2020 and 2024, led by non-invasive tests (NITs).
• Pipeline approvals expected to reduce the need for liver transplant by 35% by 2032.
🔹 Market Segmentation Analysis
By Drug ClassGLP-1 Receptor Agonists: Lead the market with 32% share, driven by strong weight-loss effects and improved insulin sensitivity, projected to surpass USD 15.8 billion by 2032.
FXR Agonists: Hold 26% share and are expected to reach USD 8.5 billion by 2032 as accelerated regulatory approvals boost adoption.
FGF21 Analogues: Account for 18% share and represent the fastest-growing segment due to their proven ability to reverse liver fibrosis.
Vitamin E & Off-label Therapies: Capture 12% share but are declining as their impact on fibrosis progression remains limited.
Combination / Others: Represent 12% share, expected to rise sharply to USD 5.2 billion by 2032 as combination therapy becomes the preferred treatment approach.
By Disease Stage
F1–F2 (early fibrosis): Dominates with 42% share as early screening initiatives and lifestyle interventions drive rapid diagnosis and treatment uptake.
F3 (advanced fibrosis): Represents 38% share with the strongest demand for targeted pharmacological therapies to prevent progression to cirrhosis.
Cirrhosis (compensated): Accounts for 20% share, supported by pipeline therapies focused on reversing cirrhosis and lowering mortality risks.
By Distribution Channel
• Hospital Pharmacies — 52%
• Retail Pharmacies — 28%
• Online Pharmacies — 20% (fastest growth at 32.5% CAGR due to home-therapy prescriptions and telehealth)
Request for Customized Sample Report as per Your Business Requirement: https://www.datamintelligence.com/customize/nash-or-mash-treatment-market
Regional Insights
United States
• Market size USD 2.15 billion in 2024 → USD 17.4 billion by 2032, CAGR 30.1%
• >12 million adults diagnosed with NASH/MASH; 80% overweight or diabetic
FDA fast-tracking breakthrough NASH drugs including FXR agonists and FGF21 analogs
Japan
• Market size USD 420 million in 2024 → USD 3.5 billion by 2032, CAGR 29.7%
• One of the highest screening rates globally, led by government-backed metabolic syndrome programs
• Major local participation in global Phase 3 NASH/MASH drug trials
Competitive Landscape
The NASH/MASH treatment market is highly competitive with rapid pipeline expansion.
Key Players
Novo Nordisk | Eli Lilly | Madrigal Pharmaceuticals | Pfizer | Gilead Sciences | Intercept Pharmaceuticals | Akero Therapeutics | Galmed Pharmaceuticals | Terns Pharma | Viking Therapeutics | Boehringer Ingelheim International GmbH., 89bio, Inc., Inventiva., CHIA TAI TIANQING PHARMACEUTICAL GROUP CO., LTD | Sagimet Biosciences
Key Highlights
• Novo Nordisk reported a 145% YoY jump in GLP-1 usage among NASH-risk populations driven by semaglutide.
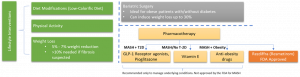
• Madrigal Pharmaceuticals became the first in class to receive FDA approval for an advanced NASH therapeutic in 2025.
• Akero Therapeutics reported fibrosis improvement in 41% of Phase 3 patients using FGF21 analog efruxifermin.
Recent Developments
• 2025 — Eli Lilly initiated Phase 3 trial combining GLP-1 + SGLT2 for accelerated fibrosis resolution.
• 2025 — Novo Nordisk announced chronic liver disease module integration in semaglutide patient management program.
• 2024 — Pfizer partnered with Japanese academic institutions for NASH biomarker validation.
• 2024 — Akero Therapeutics signed licensing agreement with Novartis to commercialize FGF21 therapy in Asia-Pacific.
Market Outlook & Opportunities
• Combination therapy projected to account for 42% of treatment revenue by 2032
• AI-based NASH risk scoring to reduce diagnostic time by 65%
• Non-invasive liquid biopsy expected to unlock USD 3.6 billion in new demand
• Asia-Pacific — fastest-growing region (31.8% CAGR) with lifestyle-disease burden and generics expansion
Buy This Report with Year-End Offer (Buy 1 report: Get 30% OFF | Buy 2 reports: Get 50% OFF each! Limited time offer): https://www.datamintelligence.com/buy-now-page?report=nash-or-mash-treatment-market
Conclusion
The Global NASH/MASH Treatment Market is entering a breakthrough commercialization phase driven by first-in-class drug approvals, weight-loss innovations, biomarkers, and combination therapy protocols.
According to DataM Intelligence, the market will expand from USD 4.85 billion in 2024 to USD 38.21 billion by 2032, transforming liver-care standards and reducing reliance on transplants.
Related Markets
Glucagon-like Peptide 1 (GLP-1) Analogues Market
Diabetes Care Drugs Market
Sai Kiran
DataM Intelligence 4market Research LLP
+1 877-441-4866
sai.k@datamintelligence.com
Visit us on social media:
LinkedIn
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.