Europe Positions as Innovation Hub in Probiotic Skincare Ingredients Market Advancing to USD 3,151.6 Million by 2035
UK and Germany Lead Regional Clean-Label Revolution as Global Market Records 12.4% CAGR Through USD 982 Million Industry
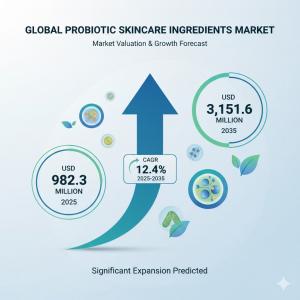
NEWARK, DE, UNITED STATES, October 27, 2025 /EINPresswire.com/ -- The global probiotic skincare ingredients market is projected to expand from USD 982.3 million in 2025 to USD 3,151.6 million by 2035, representing a compound annual growth rate of 12.4% and a substantial 3.2X increase over the forecast decade. Within this transformative microbiome skincare landscape, Europe has established itself as a strategic innovation center, driven by rigorous regulatory frameworks, sophisticated dermocosmetic traditions, and uncompromising consumer demand for vegan, clean-label, and clinically validated formulations that distinguish regional market positioning from global competitors.
European markets demonstrate compelling growth trajectories anchored by the United Kingdom advancing at 11.2% CAGR and Germany maintaining steady momentum at 8.4% CAGR through 2035. These growth rates reflect Europe's mature cosmeceutical infrastructure, where probiotic skincare ingredients benefit from established pharmaceutical-grade manufacturing standards, comprehensive clinical validation protocols, and consumer trust in scientifically substantiated microbiome-friendly claims. France contributes additional regional strength through its heritage beauty industry, reinforcing Europe's leadership in bridging biotechnology innovation with premium skincare formulation expertise.
Postbiotics Leadership Defines European Market Strategy
The postbiotics and lysates segment commands 49.5% of global market revenue in 2025, with European ingredient manufacturers driving substantial innovation through superior stability profiles, safety validation, and proven efficacy in barrier repair applications. German biotechnology firms particularly excel in developing heat-inactivated microbial fractions that deliver bioactive metabolites while eliminating viability concerns associated with live probiotic formulations. This technical sophistication enables reliable integration into mainstream cosmetic products without cold-chain logistics or specialized encapsulation technologies that constrain live probiotic adoption.
European regulatory frameworks including EU Cosmetics Regulation 1223/2009 create stringent safety and efficacy requirements that favor postbiotic ingredients with established toxicological profiles and documented skin compatibility. DSM-Firmenich, commanding 9.1% global market share, exemplifies European leadership through extensive biotechnology capabilities and strategic partnerships with premium skincare brands requiring clinically validated microbiome actives.
Vegan and Clean-Label Claims Drive European Consumer Preference
European consumers demonstrate exceptional commitment to vegan, clean-label, and fragrance-free skincare formulations, positioning the region as a catalyst for ethical innovation within the probiotic ingredients category. The UK market exemplifies this trend, where consumer preference for cruelty-free and sustainably sourced ingredients influences purchasing decisions across premium dermocosmetic and mass-market beauty segments. Probiotic ingredients derived from fermentation processes align seamlessly with vegan certification requirements, enabling brands to satisfy both microbiome efficacy and ethical positioning without formulation compromises.
Germany's stringent environmental regulations and consumer transparency expectations accelerate adoption of microbiome-friendly claims supported by comprehensive clinical documentation. European ingredient suppliers increasingly provide brands with substantiation dossiers documenting strain-specific benefits, safety profiles, and compatibility with sensitive skin conditions, addressing regulatory scrutiny while building consumer confidence.
Serums and Ampoules Dominate Premium Formulation Strategies
Serums and ampoules account for 46.5% of market share in 2025, reflecting European consumer sophistication in multi-step skincare routines and preference for concentrated, efficacy-driven formulations. German and French cosmeceutical manufacturers particularly favor these formats for delivering high-potency postbiotic lysates and prebiotic complexes that support barrier repair and hydration functions. The lightweight textures and rapid absorption characteristics of serums enable optimal delivery of microbiome actives while accommodating layering within comprehensive skincare regimens popular across European demographics.
UK indie beauty brands leverage serums as innovation vehicles for introducing novel probiotic strains and synergistic prebiotic-postbiotic complexes that address specific skin concerns including sensitivity, redness, and environmental stress. This application diversity extends beyond basic moisturization into targeted therapeutic benefits, positioning probiotic serums within dermocosmetic categories commanding premium pricing.
Barrier Repair and Hydration Functions Anchor Clinical Positioning
The barrier repair and hydration segment captures 42.4% of market value, with European dermatology research establishing scientific foundations for probiotic ingredients' role in strengthening skin barrier function and enhancing moisture retention. German dermatological institutions collaborate with ingredient suppliers to document how postbiotic lysates modulate skin microbiome composition, reduce trans-epidermal water loss, and accelerate barrier recovery following environmental insults. This clinical evidence enables European cosmetic brands to position probiotic formulations within therapeutic skincare categories addressing chronic skin conditions rather than purely cosmetic applications.
UK pharmacy channels emphasize barrier-repair claims supported by dermatologist endorsements, creating professional validation pathways that accelerate mainstream consumer adoption. The prevalence of sensitive skin concerns across European populations, exacerbated by pollution exposure and climate variability, drives sustained demand for gentle yet effective probiotic formulations that restore microbiome balance without triggering inflammatory responses. This functional positioning proves particularly effective within European markets where consumers prioritize long-term skin health over temporary aesthetic improvements.
Regulatory Rigor Creates Competitive Moats
European ingredient manufacturers benefit from comprehensive regulatory frameworks that establish quality standards while creating natural barriers to entry for suppliers lacking robust scientific documentation. The EU requirement for cosmetic ingredient safety assessments under REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation ensures that probiotic actives undergo thorough toxicological evaluation before market introduction. This regulatory rigor protects established European suppliers with extensive testing capabilities while constraining emerging competitors lacking infrastructure for comprehensive safety substantiation.
Germany's BfR (Federal Institute for Risk Assessment) guidelines for microbial ingredients in cosmetics provide additional specificity around contamination controls and preservation requirements, elevating manufacturing standards across European production facilities. These regulatory advantages extend beyond safety compliance into marketing claim substantiation, where European suppliers provide brands with clinical trial data, in vitro efficacy studies, and consumer perception research supporting microbiome-friendly positioning that withstands regulatory scrutiny.
Strategic Growth Outlook for European Markets
The forecast period through 2035 presents substantial opportunities as microbiome science transitions from niche specialty into mainstream skincare foundation. The second-half acceleration from 2030 to 2035, accounting for 64.2% of total decade growth, will be influenced significantly by European innovation in next-generation prebiotic-postbiotic complexes, personalized microbiome formulations, and digital diagnostic tools enabling customized ingredient recommendations based on individual skin microbiome profiles.
European markets offer strategic differentiation through established biotechnology infrastructure, pharmaceutical-grade manufacturing capabilities, and regulatory frameworks supporting substantiated therapeutic claims. Organizations positioning within these markets access quality-conscious beauty brands willing to invest in clinically validated ingredients as essential components of premium dermocosmetic portfolios rather than marketing-driven trend ingredients.
Get Instant Access for Only $2000 | Don’t Miss This Exclusive Offer!
https://www.futuremarketinsights.com/reports/sample/rep-gb-24917
Checkout Now to Access Industry Insights:
https://www.futuremarketinsights.com/checkout/24917
Similar Industry Reports
Probiotic Chewing Gum Market
https://www.futuremarketinsights.com/reports/probiotic-chewing-gum-market
Rahul Singh
Future Market Insights Inc.
+1 347-918-3531
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.