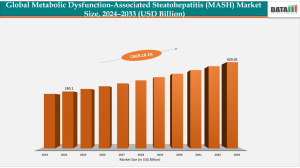
MASH Drugs Market Set to Hit $929M by 2033 Amid New Approvals and Innovative Therapies
Global MASH drugs market grows rapidly, fueled by new FDA approvals, innovative pipeline therapies, and rising obesity-driven demand.
Top Key Players – Akero, Boeringer Ingelhei, Novo Nordisk, Madrigal Pharmaceuticals.
𝗚𝗲𝘁 𝗮 𝗦𝗮𝗺𝗽𝗹𝗲 𝗣𝗗𝗙 𝗕𝗿𝗼𝗰𝗵𝘂𝗿𝗲 𝗼𝗳 𝘁𝗵𝗲 𝗥𝗲𝗽𝗼𝗿𝘁 (𝗨𝘀𝗲 𝗖𝗼𝗿𝗽𝗼𝗿𝗮𝘁𝗲 𝗘𝗺𝗮𝗶𝗹 𝗜𝗗 𝗳𝗼𝗿 𝗮 𝗤𝘂𝗶𝗰𝗸 𝗥𝗲𝘀𝗽𝗼𝗻𝘀𝗲): https://www.datamintelligence.com/download-sample/metabolic-dysfunction-associated-steatohepatitis-mash-drugs-market
United States – Industry News
✅ FDA Approves Wegovy for MASH
On August 18, 2025, the FDA approved Novo Nordisk’s Wegovy for treating noncirrhotic MASH, marking it as the second FDA-approved drug for the condition.
✅ FDA Accepts Surrogate Endpoint Proposal for MASH
On August 27, 2025, the FDA accepted a proposal for a surrogate endpoint in MASH trials, potentially expediting future drug approvals.
Japan – Industry News
✅ Inventiva and Hepalys Initiate Phase 1 Trial
In February 2025, Inventiva and Hepalys Pharma began a Phase 1 clinical trial in Japan for lanifibranor, a pan-PPAR agonist targeting MASH.
South Korea – Industry News
✅ South Korean Biotech's GLP-1 Drug Shows MASH Efficacy
On June 16, 2025, a South Korean biotech's GLP-1 candidate demonstrated reduced liver fat in MASH patients, with added weight loss benefits.
✅ Dong-A ST and Hanmi Pharm Showcase MASH Drug
On June 23, 2025, Dong-A ST and Hanmi Pharmaceutical presented advancements in MASH treatments at a major event.
Europe – Industry News
✅ EMA Grants Conditional Authorisation to Rezdiffra
On June 20, 2025, the European Medicines Agency recommended conditional authorisation for Madrigal Pharmaceuticals' Rezdiffra, the first MASH treatment in the EU.
✅ EMA Qualifies AIM-NASH AI Tool for Liver Biopsy Analysis
On March 20, 2025, the EMA qualified AIM-NASH, an AI tool to assist pathologists in diagnosing MASH from liver biopsy samples.
Looking For A Detailed Full Report? Get it here: https://www.datamintelligence.com/buy-now-page?report=metabolic-dysfunction-associated-steatohepatitis-mash-drugs-market
Key Drug Approvals
• Resmetirom (Rezdiffra): Approved in 2024, resmetirom is the first FDA-cleared treatment for MASH, targeting liver fat accumulation and inflammation.
• Wegovy (Semaglutide): In August 2025, Wegovy received accelerated FDA approval for MASH, becoming the first GLP-1 receptor agonist approved for this indication.
• Efruxifermin: Akero Therapeutics' efruxifermin has shown promising Phase 3 results, with significant improvements in liver fibrosis and cirrhosis symptoms.
Pipeline & Emerging Therapies
The MASH pipeline is robust, with several investigational therapies in various stages of development:
• Lanifibranor: An agonist of PPAR receptors, showing potential in reducing liver inflammation and fibrosis.
• Efruxifermin: A FGF21 analog demonstrating significant improvements in liver fibrosis and cirrhosis symptoms.
• GLP-1 Receptor Agonists: Drugs like Wegovy are being explored for their effects on liver fat reduction and metabolic improvements.
Market Segments:
• By Disease Stage (Intraoral X-ray, Extraoral X-ray, Imaging Software, Others)
• By Medication (2D Digital Radiography, 3D CBCT, Optical/impression scanners, Digital sensors, Hybrid Systems)
• By Route of Administration (Endodontics, Implantology, Orthodontics, Oral & Maxillofacial Surgery, Others)
Get Customization in the report as per your requirements: https://www.datamintelligence.com/customize/metabolic-dysfunction-associated-steatohepatitis-mash-drugs-market
Growth Drivers:
• Rising Obesity Rates: Approximately 2.3 billion children and adults are living with overweight or obesity, creating a direct risk for MASH.
• Advancements in Drug Development: The approval of new therapies, such as resmetirom and Wegovy, is expanding treatment options.
• Increased Awareness: Growing recognition of MASH as a serious liver disease is driving demand for effective treatments.
Regional Insights:
• North America: Dominated the market with a revenue share of 43.5% in 2024.
• Asia-Pacific: Expected to witness the fastest growth during the forecast period, driven by increasing obesity rates and healthcare access
Related Reports:
NASH/MASH Treatment Market
Melasma Treatment Market
Kailas Disale
DataM Intelligence 4market Research LLP
+1 877-441-4866
kailas@datamintelligence.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.