Dual Oral Antivirals Market Set to Reach USD 5.1 Billion by 2035 | United States Is Projected To Grow At 3.4%
Chronic Viral Suppression Segment Is Projected To Grow At A CAGR Of 4.3%, Whereas Curative/Sustained Virologic Response (Hepatitis C) Is Likely To Grow At 4.0%
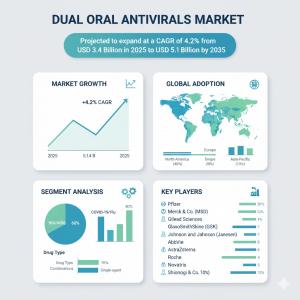
ROCKVILLE, MD, UNITED STATES, October 17, 2025 /EINPresswire.com/ -- The global Dual Oral Antivirals Market is projected to grow from a value of USD 3.4 billion in 2025 to approximately USD 5.1 billion by 2035, registering a compound annual growth rate (CAGR) of 4.2% over that period. The chronic viral suppression (HIV) therapeutic indication is dominant, holding roughly 35% of market share in 2025. Growth is being steered by increasing incidence of chronic viral infections, rising demand for simplified two-drug oral regimens, and heightened emphasis on patient compliance and resistance management. Fixed-dose combinations and long-acting oral formulations are emerging as key innovations, supported by favorable regulatory frameworks and growing investment into development and access.What’s Driving Market Growth:
Major growth levers include the burden of chronic viral diseases like HIV and hepatitis, which creates demand for treatments that are simpler, safer, and easier to adhere to long-term. Dual oral antivirals help reduce pill burden and risk of missed doses, which in turn can reduce resistance. Technological advances — particularly in co-formulation, pharmacokinetics, and long-acting delivery — support these benefits. Increased awareness among patients and providers, along with better healthcare access in emerging economies and favorable regulatory policies for fixed-dose oral combination therapies, are also enabling broader adoption.
Key Trends and Emerging Patterns:
Fixed-dose combination pills are rising in prominence, offering dual agents in one oral dose to simplify treatment. Long-acting oral regimens are being developed, sometimes enabling weekly or less frequent dosing, which helps compliance and quality of life. There is growing use of digital adherence tools (mobile apps, monitoring devices) and predictive analytics (including AI-based models) to optimize regimen selections and monitor efficacy and resistance. Companies are also investing in novel antiviral drug classes, bioavailability, and safety in dual combinations.
Challenges & Barriers:
Despite strong momentum, several obstacles remain. Development costs are high, particularly for co-formulated drugs and long-acting oral forms. Regulatory approval processes for combination therapies are complex; safety, drug-drug interaction, and pharmacokinetics must be rigorously demonstrated. Awareness and access vary widely global-ly; in many low- and middle-income regions, healthcare infrastructure, diagnostic capacity, and patient monitoring are still underdeveloped. Competition from generic single-agent antivirals, pricing pressure, intellectual property hurdles, and issues with reimbursement in some markets also pose risks to faster growth.
Regional & Country Insights:
United States is forecast to have strong but moderate growth (~3.4% CAGR), supported by advanced research infrastructure, higher patient awareness, strong regulatory paths, and early adoption of long-acting oral combinations and digital adherence support.
China is expected to grow more rapidly (~4.6% CAGR), driven by increasing healthcare investment, efforts to expand access to chronic viral therapies, local manufacturing of dual oral regimens, and government programs aimed at disease control and improved treatment adherence.
Germany shows steady growth (~2.6% CAGR), propelled by strong healthcare systems, regulatory oversight, and patient demand for convenience and safety. Fixed-dose combinations and adherence technologies feature prominently in the German market.
Key Players & Market Competition:
Major pharmaceutical companies are active in this space: Pfizer; Merck & Co. (MSD); Gilead Sciences; GlaxoSmithKline (GSK); Johnson & Johnson (Janssen); AbbVie; Roche; AstraZeneca; Novartis; and Shionogi & Co. These companies are leading in R&D for dual oral antiviral regimens, long-acting combinations, and digital adherence tools. Several are pursuing islatravir- and lenacapavir-based combination therapies, with early data showing promising viral suppression and safety profiles.
Outlook & Strategic Implications:
By 2035, dual oral antivirals are expected to reach USD 5.1 billion globally. Continued innovation — especially fixed-dose combinations and long-acting oral formulations — will be essential. Companies that can combine safety, efficacy, regulatory compliance, and patient convenience will lead. Expanding access in emerging markets, aligning pricing and reimbursement with value, strengthening patient adherence support, and managing resistance risk will be critical success factors.
From a therapeutic perspective, HIV treatment remains the largest indication, but opportunities in curative/sustained virologic response (Hepatitis C and potentially emerging viral diseases) are growing. Drug classes like nucleos(t)ide analogues are likely to maintain strong influence due to their efficacy and established safety.
Request for Discount: https://www.factmr.com/connectus/sample?flag=S&rep_id=11215
Buy Now at USD 4500: https://www.factmr.com/checkout/11215
Check out More Related Studies Published by Fact.MR Research:
Dual Migraine Treatment Market: https://www.factmr.com/report/dual-migraine-treatment-market
Dual Chamber Prefilled Syringes Market: https://www.factmr.com/report/486/dual-chamber-prefilled-syringes-market
Dual Therapy Stent Market: https://www.factmr.com/report/572/dual-therapy-stent-market
Dual Antiplatelet Therapy Market: https://www.factmr.com/report/1002/dual-antiplatelet-therapy-market
Editor’s Note
Fact.MR is a leading global market research and consulting firm, known for delivering actionable insights across industries. Our study on the Dual Antiplatelet Therapy Market integrates technology assessment, clinical trends, and regional insights to provide strategic intelligence for healthcare stakeholders. As innovation accelerates in vascular access technologies, Fact.MR continues to guide market participants in capturing opportunities and navigating challenges in this rapidly advancing field.
S. N. Jha
Fact.MR
+1 628-251-1583
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.