CounterAct Completes Human Factors Engineering/Usability Engineering (HFE/UE) Formative Study with Positive Results
A Human Factors Engineering/Usability Engineering (HFE/UE) formative study was conducted to support the design and development of the CounterAct System.
VISTA, CALIFORNIA, USA, May 15, 2023/EINPresswire.com/ -- CounterAct’s combination rescue medication platform device completed a Human Factors Engineering/Usability Engineering (HFE/UE) formative study. The purpose of the study was to support the design and development of the CounterAct System and served to identify use related risks and risk control measures. The study was conducted in accordance with FDA and industry guidance [Ref. ANSI/AAMI HE75:2009/(R) 2018, IEC 62366-1:2015/AMD1:2020].
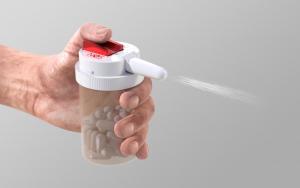
The CounterAct System contains a spring-loading trigger and a single dose of naloxone or other rescue medication, with a nozzle for delivery into the nostril. With the push of a button, the rescue medication sprays into the nostril. Because the CounterAct Cap snaps directly onto a typical prescription pill bottle, the rescue dose of medication is always near the source of the drug, keeping it readily available for use by anyone near the victim. The FDA has confirmed that the 505(b)(2) pathway is appropriate for the CounterAct System.
In this formative study, 15 participants who are representative of the intended user population based on previous P-IND FDA meeting feedback, were evaluated on their ability to use the CounterAct System without use errors, close calls, operational difficulties or intentional misuse, and on their ability to demonstrate comprehension of the instructional materials by responding to specific knowledge-based questions. Ages ranged from 10 – 71 years old.
All participants were provided a high-level overview of the CounterAct System, provided the CounterAct System and associated instructions for use, directed to a mannequin, asked to imagine that they had walked into an overdose situation and asked to use the CounterAct System as if it was a real-life situation. After a discussion related to their experience, participants were asked to use the CounterAct System a second time. In the formative usability study, all participants were untrained and simply provided the CounterAct System. Training was not provided in order to pose the most challenging and real-life scenario.
The results revealed that 15 of 15 participants successfully used the CounterAct System (i.e., removed cap, unfolded nozzle, inserted nozzle into one nostril and pressed the activation button) at least once. 15 of 15 participants successfully understood the information provided within the Instructions for use (i.e., when to call 911, when/if to administer a 2nd dose of naloxone. 5 of 15 participants were unsure how far to insert the nozzle and were concerned that inserting it too far may hurt the person. Note, all participants were successful as they inserted the nozzle tip, at least into the entryway of the nostril. Average time for each participant to encounter the mannequin and to correctly use and administer the Counteract System was 18.6 seconds.
Co-Founder, Dr. Todd Pizitz, commented, “We developed this rescue medication device because we know an overdose, seizure, or some sort of anaphylactic reaction can occur, and the counter agent is not always nearby. Pairing the prescription medication with the rescue medication in one device has been our mission to help save lives from opioid overdoses and other related emergency medical situations. We are pleased with the results of the HFS.”
Post HFS study discussion revealed that all 15 participants stated that they would want the CounterAct System as part of prescribed opioid medication, and believed that it would reduce overdose fatalities due to having an immediately available and accessible treatment option.
The CounterAct System has been issued both US and Canadian patents, and the team continues to further device development.
Todd D. Pizitz, PhD
CounterAct
+1 619-507-7907
email us here
Visit us on social media:
LinkedIn
Other
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.