The lipid membranes are druggable and available for AI-based drug design (acticle by Receptor.AI)
Article 2: https://doi.org/10.1016/j.bbadis.2022.166614
Are the lipid membranes druggable?
Transmembrane proteins constitute a significant portion of the most promising targets in the human proteome. They include receptors, ionic channels, transporters, adhesive and structural proteins, which took part in the majority of important cellular signalling and metabolic pathways.
The usual way of targeting transmembrane proteins with small molecules is the same as for water-soluble ones: one develops a drug, which binds to a water-exposed active site on either extracellular or intracellular parts of the protein. This means that the most distinctive feature of the membrane proteins, namely their location in the lipid bilayer, is ignored.
Indeed, modulation of the lipid environment may strongly change the protein activity. The physical state and composition of the lipid bilayer influence the lateral mobility of the protein in the membrane, its conformational dynamics and the flexibility and accessibility of the binding site. Despite this, the lipid bilayer is almost never considered a druggable entity by itself in modern drug discovery.
Receptor.AI is working on a novel way of targeting membrane proteins involving their lipid environment as a first-class druggable entity. We believe that the activity, selectivity and safety of the drugs, which bind to the membrane proteins, could be greatly improved by means of double targeting: acting on the water-exposed active sites and the membrane environment of the protein at the same time.
Until recently, there were no convincing proofs of this concept, but the recent research of the CSO of Receptor.AI Dr. Semen Yesylevskyy, provided confidence in the correctness of our assumptions.
The first evidence was obtained last year. The ligand binding propensity of the platelet membrane receptor P2Y12 was shown to be strongly dependent on the membrane composition and ordering. Moreover, one of the widespread anti-platelet drugs - ticagrelor - appeared to be strongly membranotropic and able to modulate the membrane environment and, thus, the binding propensity of its own target protein.
However, the druggability of the lipid membrane itself remained questionable until recently. The recent study, co-authored by the CSO of Receptor.AI Dr. Semen Yesylevskyy, demonstrated that the lipid bilayer of the plasma membrane is druggable and suitable for facilitating the selective delivery of drugs to cancer cells.
The biggest challenge of targeted cancer therapy is achieving high toxicity of drugs to the tumour while ensuring safety for normal cells. Currently, this selectivity is based solely on targeting specific proteins which are overexpressed or otherwise misregulated in cancer cells.
However, the molecular differences between normal and cancer cells are not limited to proteins. The lipid membrane composition of cancer cells differs drastically from their normal counterparts, but this difference is rarely considered as an option for selective drug delivery.
The abovementioned work has shown that amphiphilic nanoparticles made of gemcitabine-squalene conjugates accumulate selectively in the plasma membranes of malignant cells. This facilitates their uptake by the cancer cells and causes their death, while the normal cells survive in the same conditions.
This study is convincing proof of the concept that dedicated membranotropic drugs could be designed to target the lipid bilayers of malignant cells. The AI-based technology of creating various types of membranotropic drugs is currently under active development in Receptor.AI.
Receptor.AI approach to membranotropic drug discovery
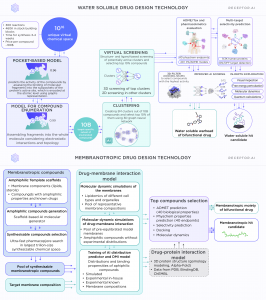
Our approach is based on using the capabilities of the Receptor.AI drug discovery platform, which designs two separate active chemical moieties for water-exposed active sites of the membrane protein and for the membrane itself or the intramembrane regions of the protein of interest.
The water-soluble compound is designed using our well-tested production workflow, schematically shown in Fig. 1.
The membranotropic moiety is constructed by a separate drug-membrane interaction (DMI) AI model. This model is developed and trained on the dataset, which is compiled from the data of extensive molecular dynamics simulations and wet lab experiments (Fig. 1).
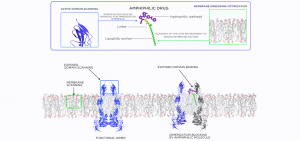
The membranotropic drugs are intended to modulate protein conformation and dynamics and/or impose an allosteric effect on the remote active sites. We consider several modes of action for such compounds:
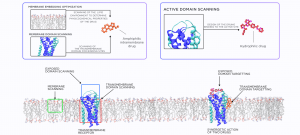
1. Direct specific interaction with the intra-membrane parts of the protein. The drug binds to the lipid binding pockets, lipidated residues or other distinct structural features of the hydrophobic intramembrane surface.
2. Direct nonspecific interaction with the intra-membrane parts of the protein. The drug binds to the protein-lipid interface in general without strict structural specificity.
3. Indirect influence by modification of the lipid bilayer. The drug changes ordering, fluidity, viscosity or other physical properties of the lipid bilayer, which influences the protein indirectly.
4. PPI blocking. Blocking homologous (dimerization) or heterologous interactions of the membrane proteins with other proteins (either membrane or soluble) by preventing contacts inside the membrane or close to the membrane surface.
5. The membranotropic drugs could be designed as bifunctional molecules - the amphiphilic molecules, which are incorporated into the lipid bilayer by one end and bind to the desired water-exposed active site of the protein by the other end (Fig. 3).
Another possibility is to develop separate water-soluble and membranotropic drug molecules which work in a synergistic way. A conventional water-soluble drug is developed for the water-exposed binding site of the protein, while the membranotropic compound is developed separately and targets the membrane environment of the protein of interest (Fig. 4).
We strongly believe that the combination of AI technologies with the idea of targeting membrane proteins simultaneously with modulating their membrane environment will lead to a breakthrough in drug discovery for membrane-associated targets.
If you have a promising membrane protein and you want to be among the first who benefit from this novel technology, contact us right now!
Alan Nafiiev
Receptor.ai
15172837276398
ai@receptor.ai
Visit us on social media:
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.