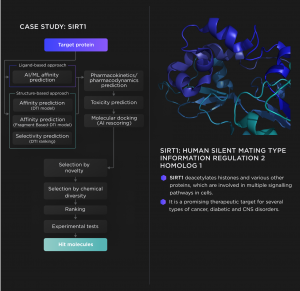
Developing anticancer inhibitors of SIRT1 with RECEPTOR.AI SaaS Platform
Our AI-based drug discovery platform allows virtual screening of billions of chemical compounds in just a few hours against the protein target of interest to find the molecules with the best binding propensity, which are called hits.
The platform is fully automated and combines 40+ integrated AI models with in silico approaches, such as AI-assisted molecular docking. Our technique ensures the identification of high-quality hits, which are favourable candidates for further lead discovery and optimisation. Our partnership with top biotech companies allows us to synthesise and experimentally evaluate hit compounds alongside in silico predictions to provide precise and reliable results.
Application of RECEPTOR.AI SaaS platform
The Receptor.AI SaaS platform allows the user to automate small molecule drug discovery workflow. The platform is modular and fully configurable, allowing the construction of a drug discovery R&D pipeline which meets any specific needs of the client (integration of the custom-tuned AI models, incorporation of chemical databases and custom datasets, etc.).
Receptor.AI SaaS platform currently implements the phase of hit discovery and performs two-stage virtual screening: initial screening of the large chemical spaces followed by the precise secondary screening within a preselected pool of compounds. The platform assesses the compounds based on their predicted affinities and pharmacokinetic profiles. The next version of the platform will include lead discovery and lead optimisation modules as well.
Initial screening
The system utilises ligand-based and two structure-based screening techniques in parallel to get the most reliable virtual screening consensus scores. There are more than 35 pharmacokinetic predictive endpoints to choose from, which could be used to select the molecules with desirable ADMET properties. This allows for a much stricter selection of compounds with high predicted affinity, selectivity and activity while ensuring that they are safe, non-toxic and favourable in terms of ADMET parameters.
Secondary screening
The system performs fully automated AI-assisted molecular docking of the molecules selected in the initial screening phase. It prioritises the most promising candidates for further synthesis and biological validation. At this stage, the proteome-wide binding profiles are also analysed to identify possible off-target interactions and to get a reliable selectivity prediction.
Technological innovations of Receptor.AI allowed finding novel inhibitors of BRD4 in less than 6 weeks, including their synthesis and biological validation.
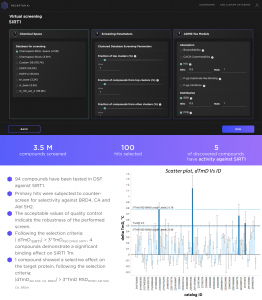
Enamine stock database of 3.5M high-quality drug-like molecules was screened using the Drug-Target Interactions (DTI) models integrated into the SaaS platform. After that, our proprietary ADME-Tox models were used to narrow the results with the following ADMET endpoints: Ames mutagenicity, carcinogenicity (ISF and OSF), DILI, hERG blocking, Bioavailability, HIA, BBB crossing, PPB, P-gp and CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP3A4 substrate-like binding/inhibition. 21556 best-ranked compounds were advanced to the secondary screening phase, where they were evaluated by Molecular Docking with a custom-tuned AI-based scoring function.
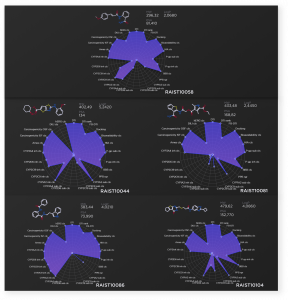
Predicted ADMET profiles and activities of experimentally validated hit compounds
RAIST10058. This compound has shown an outstanding nanomolar activity (90 nM) and high selectivity, which makes it de facto a lead compound. Moreover, our multi-task ADMET model predicted a high propensity for BBB permeation for this compound, low hepatotoxicity and an acceptable plasma protein binding rate. The lack of predicted degradation by cytochromes needs further optimization, but this is not critical in the hit discovery stage.
RAIST10044. This compound has also shown good experimental activity. It has a promising bioavailability/toxicity profile and prospects of being biodegradable by cytochromes. But relatively high hepatotoxicity and PPB still need optimization.
RAIST10081. This compound has good experimental activity and a good toxicity profile, but it is less biodegradable by cytochromes in comparison to the RAIST10044. It also has higher PPB and possesses a moderate risk of DILI induction.
RAIST10086. This compound has good experimental activity and a good toxicity profile, but it is even less biodegradable by cytochromes, lacks the BBB permeation, possesses a moderate risk of DILI induction and has a somewhat questionable distribution due to relatively high PPB.
RAIST10104. This compound has shown decent experimental activity. It belongs to a novel class of compounds which were previously unknown as SIRT1 inhibitors. It has a good toxicity profile, but there are questions concerning its cytochrome safety, DILI and PPB. However, these disadvantages are acceptable on the hit discovery stage, taking into account its unprecedented novelty.
Details of experimental validation
100 best-ranked hit compounds were experimentally validated by our partners. 5 of them have shown high affinity to SIRT1. These compounds are currently subject to in-depth experimental validation followed by hit-to-lead and lead optimisation stages.
No alt text provided for this image
Alan Nafiiev
Receptor.AI
+49 1517 2837276
ai@receptor.ai
Visit us on social media:
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.