The Rapid Influenza Diagnostic Tests Market to Witness Decisive Innovation
The market is expected to top US$ 2 Bn by 2031, which reflects a CAGR of around 8.7% for the decade.
PUNE, MAHARASHTRA, INDIA, February 15, 2022 /EINPresswire.com/ -- The Rapid Influenza Diagnostic Tests Market is ascertained to make greater strides in the future. The present-day and futuristic cutting-edge technology, namely IoT, AI, and Big Data operate better in a lightning-fast and reliable internet connection. The benefits of high-speed internet would be seen in telecare in the next 2 to 3 years, but going forward, more authentic data streams are likely to come up with better-connected devices, thereby revolutionizing the healthcare system.
As per Persistence Market Research’s latest revised industry analysis, the global rapid influenza diagnostic tests market is expected to witness high growth during the forecast period. The market is expected to top US$ 2 Bn by 2031, which reflects a CAGR of around 8.7% for the decade.
Influenza is recognized as a major source of illness and mortality in humans, prompting the development and implementation of diagnostics aimed at decreasing the health and economic consequences. Influenza causes serious illness or death, primarily in high-risk people.
With the start of the flu season comes an increase in antibiotic use, elevating the danger of antibiotic resistance in the body. The speedy turn-around times of devices used at point-of-care aid in the preparation of a targeted and successful treatment plan based on test results. Rapid influenza diagnostic tests have a short turnaround time, with some offering results in under 15 minutes. As a result, the adoption of rapid influenza diagnostic tests helps prevent the over-prescription of antibiotics as well as avoid the core cause of bacterial resistance and its negative consequences.
Get Sample Copy of this Report@ https://www.persistencemarketresearch.com/samples/31040
The current COVID-19 pandemic has also boosted the demand for rapid influenza diagnostic tests. When compared to influenza, COVID-19 has a similar illness presentation – both viruses induce respiratory problems. Furthermore, both are spread via touch, droplets, and fomites. COVID-19 patients have been misdiagnosed with influenza in the past, which has increased the demand for virus rapid detection kits.
The emergence of smartphone-based disease screening has increased market revenue. The Human Genome Project and breakthroughs in molecular and biomedical technology have led to the creation of a plethora of assays and technologies useful for the diagnosis and monitoring of influenza infections. These new technologies, which are based on genomic (PCR-based) and proteomic (microarray-based detection) techniques, aid in the discovery of novel influenza viruses. They also allow for improved surveillance and rapid detection of infectious diseases, which presents a good business opportunity for market players.
Company Profiles:
Thermo Fisher Scientific
Hologic
Quidel Corporation
F. Hoffmann-La Roche AG
Abbott Laboratories
Becton Dickinson and Company
Danaher Corporation
Meridian Bioscience
bioMérieux SA
Luminex Corporation
Siemens Healthineers AG
GenMark Diagnostics
Sekisui Diagnostics
LLC altona Diagnostics GmbH
SA Scientific
ELITech Group
Request for Methodology@ https://www.persistencemarketresearch.com/methodology/31040
Key Takeaways from Market Study
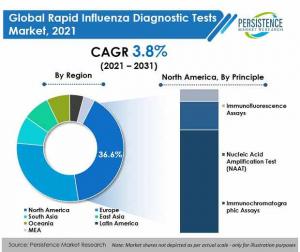
By principle, immunochromatographic assays are set to hold a high share of 66.8% in 2021, expanding at 6.1% CAGR over the forecast period.
By test type, the influenza A+B test is expected to hold a 79% market share and is expected to continue growing at a similar trend due to its benefits such as being simple to use and rapid diagnosis.
By sample, the nasopharyngeal swab segment is expected to 31% market share, and nasopharyngeal aspirate is the second-leading segment, which accounts for 27.5%.
By end-user, office-based settings account for nearly 22.5% market share.
By region, North America held 36.6% of the global market share in 2021.
“Rising demand for rapid disease diagnosis, increasing influenza prevalence, and increased research for diagnostic technologies are expected to provide growth opportunities for market players,” says an analyst of Persistence Market Research.
Market Competition
Leading market players are focusing on product approvals and launches as a key growth strategy for global expansion, thereby enhancing their market presence. They are also emphasizing on entering into strategic partnerships with local suppliers and distributors to expand product reach.
In March 2021, Abbott received an Emergency Use Authorization (EUA) from the US Food and Drug Administration (FDA) for its AlinityTM m Resp-4-Plex molecular assay, which can identify and discriminate SARS-CoV-2, influenza A, influenza B, and respiratory syncytial virus (RSV) all in one test. This test has received CE certification and is available in countries other than the United States.
In February 2020, the FDA granted Quidel an Emergency Use Authorization (EUA) to market its Sofia® 2 Flu + SARS Antigen FIA for the rapid, simultaneous qualitative identification, and characterization of nucleocapsid protein antigens from SARS-CoV-2, influenza A, and influenza B in direct nasopharyngeal (NP) and nasal (NS) swab samples from persons suspected of respiratory viral infection consistent with COVID-19.
In August 2017, Meridian Bioscience, Inc. announced the expansion of its ImmunoCard STAT!® product line with the addition of ImmunoCard STAT! FLU A&B.
Access Full Report@ https://www.persistencemarketresearch.com/checkout/31040
What Does the Report Cover?
Persistence Market Research offers a unique perspective and actionable insights on the rapid influenza diagnostics tests market in its latest study, presenting a historical demand assessment of 2016 – 2020 and projections for 2021 – 2031.
The research study is based on the principle (immunochromatographic assays, immunofluorescence assays, and nucleic acid amplification test (NAAT)), test type (influenza A test, influenza B test, and influenza A+B test), sample (throat swab, nasal swab, nasal aspirate, nasal wash, nasopharyngeal swab, nasopharyngeal aspirate, nasopharyngeal wash, and others) and end-user (hospitals, diagnostic centers, nursing homes, office-based settings, urgent care centers, retail pharmacy clinics, schools & universities, public health camps and others (cruise ships, correctional facilities, etc.) in seven prominent regions.
Access Related Reports-
Trichomonas Rapid Testing Market: Persistence Market Research published a report on the trichomonas rapid testing market, which considers the global industry analysis 2014 – 2018 and opportunity assessment 2019-2029, and projects that the trichomonas rapid testing market is expected to reach ~ US$ 360 Mn by the end of 2019 in terms of value, and is forecasted to reach ~ US$ 590 Mn by the end of 2029.
Molecular Diagnostics Reagent Market: The global molecular diagnostic reagent market is expected to be driven by the positive research and development present. The National Institute of biologics designed for in-vitro diagnostics for HIV virus, hepatitis virus issues great interest in clear molecular diagnostic reagents for better test results.
About Us:
Persistence Market Research (PMR), as a 3rd-party research organization, does operate through an exclusive amalgamation of market research and data analytics for helping businesses ride high, irrespective of the turbulence faced on the account of financial/natural crunches.
Atul Singh
PMR
+1 646-568-7751
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.