Isolere Bio Enters into Research Collaboration Agreement with Oxford Biomedica
to Develop Enabling Manufacturing Technology for Lentiviral Vectors
DURHAM, NC, US, January 31, 2022 /EINPresswire.com/ -- Isolere Bio, a bioprocessing company that provides a platform technology for tackling downstream inefficiencies in the manufacturing of biologics and Oxford Biomedica (LSE:OXB), a leading gene and cell therapy group, announced today the signing of a research collaboration agreement to develop next generation manufacturing solutions for lentiviral vectors, an industry that is seeing tremendous growth and bottleneck pressures.
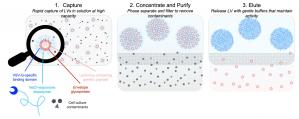
The Oxford Biomedica lentiviral platform technology was used in the first FDA and EMA approved CAR-T cell therapy. By bringing together both companies’ technologies, the research collaboration aims to develop an easily scalable purification process for lentiviral vectors with significantly improved yields and vector quality. The teams will also explore impacts of Isolere’s reagent on storage and stability. This research collaboration will focus on the development of a cutting edge lentivirus manufacturing method that involves Isolere’s proprietary IsoTag™ reagents and accompanying processes that couple affinity capture and phase separation with scalable filtration.
“Isolere is thrilled to have the opportunity to collaborate with Oxford Biomedica to apply our IsoTag technology to improving the manufacturability of lentiviral vectors. By bringing together Isolere’s unique technology and deep expertise in phase separation with Oxford Biomedica’s unparalleled capabilities in scalable manufacturing and supply of high quality viral vectors, we feel this Research Collaboration is positioned for high impact and success.” said Co-Founder and Chief Executive Officer Dr. Kelli Luginbuhl.
Oxford Biomedica’s Contract Development and Manufacturing Organization (CDMO) business provides world-leading scale-up solutions and commercial supply to pharmaceutical and biotech companies in the fast-growing viral vector industry. Isolere brings a technology with potential for transformative and highly impactful changes to the downstream manufacturing paradigm. “This collaboration highlights the true potential of the Company’s technology as a platform in biologics manufacturing, as we expand our current focus, which is on AAV, to broader pain points across the viral vector industry,” said Co-Founder and Chief Executive Officer Dr. Kelli Luginbuhl.
About Isolere Bio
Isolere Bio, Inc. is a privately held, emerging bioprocessing company developing a transformative platform technology for the next generation manufacturing of complex biologics. The company has deep expertise in the phase separation phenomena evolved by nature to perform highly specific separations in complex environments. Isolere’s development and application of phase separating proteins is backed by decades of research and high-impact publications by the company’s Co-Founders and Chief Scientific Officer. Isolere has entered into a number of collaboration agreements to externally validate its adeno-associated virus (AAV) technology and also recently announced an agreement with Aldevron to develop scalable manufacturing of its reagents. Isolere is based in Durham, North Carolina in the United States. Further information is available at www.isolerebio.com
MEDIA CONTACT: press@isolerebio.com
INVESTOR CONTACT: kluginbuhl@isolerebio.com
About Oxford Biomedica
Oxford Biomedica (LSE: OXB) is a leading, fully integrated, gene and cell therapy group focused on developing life changing treatments for serious diseases. Oxford Biomedica and its subsidiaries (the "Group") have built a sector leading lentiviral vector delivery platform (LentiVector®), which the Group leverages to develop in vivo and ex vivo products both in-house and with partners. The Group has created a valuable proprietary portfolio of gene and cell therapy product candidates in the areas of oncology, CNS disorders and liver diseases. The Group has also entered into a number of partnerships, including with Novartis, Bristol Myers Squibb, Sio Gene Therapies, Orchard Therapeutics, Santen, Beam Therapeutics, Boehringer Ingelheim, Arcellx and Cabaletta Bio, through which it has long-term economic interests in other potential gene and cell therapy products. Additionally, the Group has signed a 3-year master supply and development agreement with AstraZeneca for large-scale manufacturing of the adenoviral based COVID-19 vaccine, AZD1222. Oxford Biomedica is based across several locations in Oxfordshire, UK and employs more than 740 people. Further information is available at www.oxb.com
Kelli Luginbuhl
Isolere Bio
+1 719-322-4394
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.