Synthetic antibody demonstrates Omicron detection along with all other variants of concern
MIP Diagnostics' synthetic SARS-CoV-2 antibody shown to detect the Omicron variant as well as previously proven Alpha, Beta, Delta and Gamma variants
BEDFORD, BEDFORDSHIRE, UNITED KINGDOM, January 13, 2022 /EINPresswire.com/ -- MIP Diagnostics Ltd. has today announced that its synthetic SARS-CoV-2 antibody (COVID-19 nanoMIP) can detect the Omicron variant as well as previously proven Alpha, Beta, Delta and Gamma variants of the COVID-19 virus.MIP Diagnostics, the leading manufacturer of molecularly imprinted polymers (MIPs) - commonly termed synthetic antibodies - has demonstrated that its COVID-19 nanoMIP can detect the increasingly dominant Omicron variant. The synthetic antibody has already been shown to detect the other variants of concern - Alpha, Beta, Delta and Gamma.
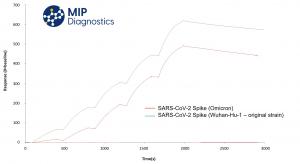
Initial assessment using pharmaceutical grade molecular modelling software demonstrated the COVID-19 nanoMIP should be able to detect the Omicron variant, and this has now been confirmed via laboratory testing. The COVID-19 nanoMIP was shown to detect the SARS-CoV-2 Omicron variant spike protein (Native Antigen Company) in buffer using surface plasmon resonance (SPR), with a magnitude of specific response comparable to other variants of the virus previously tested. Third party validation in a separate sensor device will be carried out shortly.
Originally developed in under 8 weeks, the MIP Diagnostics COVID-19 nanoMIP offers IVD manufacturers a host of benefits including high selectivity and sensitivity, demonstrated to the picogram level in a number of COVID-19 sensor devices. The robust nature of MIPs, when compared to antibodies, will also provide the superior shelf life and storage properties required by the IVD market as self-testing becomes more prevalent across multiple disease states following the COVID-19 pandemic.
Speaking on the new data, Alan Thomson, CTO at MIP Diagnostics said, “In pandemic situations, a fast response is essential, and our advanced molecular modeling software had already been utilised to assess the performance of the COVID-19 nanoMIP against the Omicron variant in-silico. This new data has confirmed our initial findings, and not only demonstrates the capabilities of the COVID-19 nanoMIP, but also supports the wider progress toward robust, non-animal derived reagents in the IVD industry.”
Keli Stockbridge
MIP Diagnostics
+44 1234 589725
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.