NX Prenatal Announces Initiation of a Clinical Study for the Detection of Placenta Accreta
Pregnant Moms with Prior C-Sections or Other Surgical Procedures at Heightened Risk
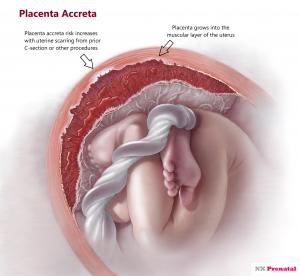
LOUISVILLE, KY, UNITED STATES, April 1, 2021 /EINPresswire.com/ -- NX Prenatal Inc. (the “Company” or “NX Prenatal”), is pleased to announce it is a sponsor and collaborator for an upcoming clinical study whereby its NeXosome® liquid biopsy platform will be key in the detection of biomarkers that can identify pregnant patients with Placenta Accreta Spectrum (PAS). This work will be pursued in collaboration with Brigham and Women’s Hospital, Boston, MA.PAS is a condition where the placenta is abnormally attached to the uterus and does not easily deliver after the infant is born. As the name implies, PAS can range in severity from abnormal adherence to invasion through the uterus and into other structures such as the bladder or pelvic blood vessels. Women with PAS can experience rapid and catastrophic bleeding, severe infection from placenta retained in the uterus, multiorgan failure and death, making it a significant contributor to maternal morbidity and mortality worldwide.
PAS is due to an imbalance in cellular regulation between the trophoblastic cells in the developing placenta and uterine lining resulting in uninhibited invasion of early placental cells. NX Prenatal has shown that variation in Circulating Microparticle (CMP) proteomic profiles may predict other obstetric conditions, including preterm birth and preeclampsia, but this approach has not yet been investigated in PAS. CMPs contain a variety of cellular material such as genetic information and proteins which have great potential to harbor a highly specific and high-intensity signal for PAS biomarkers. Therefore, the aims of this study are to identify a group of CMP-associated proteins whose profiles are different in patients with PAS compared to those without PAS, and to examine whether CMP-associated proteins can distinguish subgroups of PAS. If successful, this approach can lead to the commercialization of a biomarker panel comprised of CMP-associated proteins to predict PAS in order to ensure that affected patients receive the proper and necessary expert care.
“Our innovative technology has revealed new libraries of early warning blood-based biomarkers for preterm birth and preeclampsia, so we are compelled to utilize our platform to seek the best outcomes for pregnant patients that may be affected by placenta accreta,” noted NX Prenatal’s CEO, Brian Brohman. “Pregnant moms that have had prior C-sections, or IVF-assisted conception, or prior D&C procedures are known to be in elevated risk categories for PAS, but there is no current blood test that can help identify which of those moms may experience this life-threatening condition.”
About NX Prenatal
NX Prenatal Inc. is a private, US-based molecular diagnostics company recognized for its innovative work in new exosome-based liquid biopsy tests for the large maternal-fetal medicine market. The company's proprietary NeXosome® platform is being utilized to develop enabling, early warning systems for pregnancies that may result in spontaneous preterm birth, preeclampsia and other adverse outcomes. For more information, please visit the company's website at www.nxprenatal.com.
NX Prenatal Inc
info@nxprenatal.com
Investor and Media Relations
Visit us on social media:
Twitter
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.