SpineWelding AG Receives FDA Clearance
First 510(k) clearance for pedicle screw implant enabled by the proprietary BoneWelding technology
SCHLIEREN, SWITZERLAND, August 8, 2019 /EINPresswire.com/ -- SpineWelding AG announced today that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Elaris Pedicle Screw System, which uses the patent protected BoneWelding process. The Elaris Pedicle Screw System is intended to provide immobilization and stabilization of spinal segments through posterior, non-cervical, pedicle fixation in skeletally mature patients as an adjunct to fusion in the treatment of the following indications:• degenerative disc disease (defined as back pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies),
• spondylolisthesis, trauma (i.e., fracture or dislocation),
• spinal stenosis, curvatures (i.e., scoliosis, kyphosis, or lordosis),
• tumor, pseudarthrosis,
• and/or failed previous fusion.
The Elaris Pin polymer can be ultrasonically deposited through the Elaris Pedicle Screw in skeletally mature patients as an adjunct to fusion for the indications listed above.
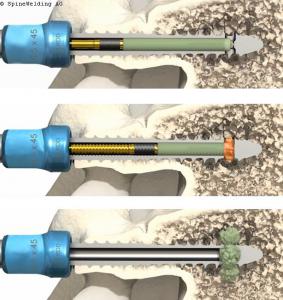
In the Elaris Pedicle Screw System, the Elaris Pin is made of a biocompatible and fully bioresorbable Poly-L-lactide-co-D, L-lactide (PLDLLA). The Elaris Pin can be inserted intraoperatively into the Elaris screw’s cannulation. Using the BoneWelder®, mechanical pressure and ultrasonic vibrations are applied during a maximum of 8 seconds to the Elaris Pin through the Elaris Handpiece connected to the pedicle screw head resulting in the ultrasonic heating of the polymer so that it melts and is extruded out of the fenestrations of the screw.
An automatic spring mechanism in the Elaris Handpiece supplies consistent force to extrude the polymer from these distal openings into the surrounding cancellous bone (indicated by the orange color in the image).
The liquid polymer solidifies immediately upon contact with bone, forming a confined, solid ring around the tip of the screw. The entire process lasts only a few seconds. Because the polymer is bioresorbable, it undergoes the natural physiologic process of hydrolysis, and is gradually metabolized into H2O and CO2.
Bench and animal testing have demonstrated that the polymer enhancement provides appropriate dynamic pullout strength for up to 52 weeks as well as improved resistance to toggle loosening compared to screws without additional fixation. Testing also has demonstrated that the pedicle screws can be removed as needed after polymer enhancement.
“The FDA clearance of the Elaris Pedicle Screw System marks a significant milestone for SpineWelding AG, demonstrating that the BoneWelding technology platform provides an adjunct to fusion in skeletally mature patients for the screw’s cleared indications”, says Dr. Joerg Mayer, Managing Director of SpineWelding AG.
“The Elaris system with ultrasonic fixation provides an adjunct to fusion in the pedicle screws in skeletally mature patients where additional fixation is needed. The ability to make an intra-operative decision to ultrasonically provide adjunctive fixation to individual screws with a seamless process, without disrupting the flow of the case is extremely attractive to surgeons,” says Dr. Frank M. Phillips – Rush University Medical Center (Chicago, IL, USA).
About SpineWelding AG
SpineWelding AG is a Swiss medical device company developing implant solutions for the human spine using the patent protected BoneWelding technology. The BoneWelding technology platform is designed to provide benefits for load-bearing implants, alongside its out-licensed applications in cranio-maxillofacial, foot & ankle, hand & wrist and dental surgery.
SpineWelding is headquartered in Schlieren, Switzerland, and is a spin-off of WoodWelding SA (Switzerland). It includes a full engineering team with an extensive background in biocompatible materials, ultrasonic process engineering and implant design, and benefits from comprehensive input from top orthopedic key opinion leaders in USA and Europe. Its Elaris Pedicle Screw System is the first product of its portfolio to receive FDA 510(k) clearance.
Prof. Dr. Gerhard Plasonig
SpineWelding AG
+41 76 366 41 55
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.