North America and Europe Sterile Injectables CMO Market Set to hit $22.37B by 2033 Amid Strong Outsourced Manufacturing
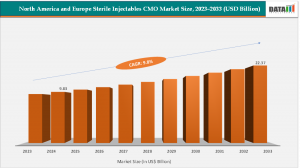
Sterile Injectables CMO Market Strengthens in North America & Europe With 9.8% CAGR to 2033
According to DataM Intelligence, the North America and Europe Sterile Injectables CMO Market reached USD 9.83 billion in 2024, up from USD 9.15 billion in 2023, and is projected to grow to USD 22.37 billion by 2033, registering a CAGR of 9.8% during 2025–2033.
According to a recent NCBI analysis, the estimated manufacturing cost for sterile vaccine formulation is approximately US$0.12 per ampoule and about US$0.35 per vial.
Norbrook in Northern Ireland has enhanced its sterile injectable production capabilities through a £1.3 million investment, adding a new modular cleanroom and upgraded HVAC systems to meet the latest EU GMP Annex 1 standards. The upgraded facility supports the manufacture of critical sterile injectable veterinary medicines.
Simtra BioPharma Solutions formerly known as Baxter BioPharma has invested more than US$250 million to expand its sterile fill-finish operations in Bloomington, Indiana, driven by the rising demand for biologics and injectable therapeutics.
Download PDF Brochure:
https://www.datamintelligence.com/download-sample/north-america-and-europe-sterile-injectables-cmo-market
Browse in-depth TOC on "North America and Europe Sterile Injectables CMO Market"
68 – Tables
62 – Figures
188 – Pages
Key Highlights from This Report
1. North America remained the leading region in the sterile injectables CMO market, accounting for 59.51% of total revenue in 2024.
2. On the basis of therapeutic area, the oncology segment emerged as the top contributor, capturing 32.39% of the market revenue in 2024.
Growth Drivers
1. Over 45% of new drug approvals in 2024 were sterile injectable formulations, particularly biologics and oncology drugs.
2. Global biopharmaceutical R&D investment reached USD 280 billion in 2024, with over 35% allocated to injectable therapeutics.
3. More than 320 sterile injectables facilities in North America & Europe reported capacity constraints in 2024, increasing outsourcing reliance.
4. The injectable pipeline expanded from 4,900 candidates in 2023 to 6,100 in 2024, pushing demand for high-throughput fill–finish capabilities.
5. Automated & robotics-enabled fill–finish systems adoption grew 70% YoY, improving sterility assurance and reducing production errors.
Market Segmentation Analysis
By Service
Aseptic Fill–Finish Services lead with 55% share (USD 10.1 billion in 2024), projected to reach USD 32 billion by 2032 at 16.2% CAGR.
Lyophilization Services account for 25% share, growing at 14.8% CAGR.
Analytical & Packaging Services represent the remaining 20%.
By Molecule Type
Biologics dominate with 58% share, driven by monoclonal antibodies and cell/gene therapies.
Small Molecules hold 42%, driven by oncology and anti-infectives.
By Dosage Form
Vials represent 65% share, remaining the preferred sterile format.
Prefilled Syringes growing fastest at 18% CAGR, supported by self-injectable biologics.
Request for Customized Sample Report as per Your Business Requirement: https://www.datamintelligence.com/customize/north-america-and-europe-sterile-injectables-cmo-market
Regional Insights
North America
Valued at USD 11.2 billion in 2024, projected to reach USD 35.6 billion by 2032 at 59.51% CAGR.
The U.S. FDA approved 52 sterile injectables in 2024, boosting CMO demand.
Over 60% of U.S. biotech startups outsource sterile fill–finish operations.
Major expansions announced across New Jersey, Indiana, and Ontario facilities.
Europe
Valued at USD 7.2 billion in 2024, expected to reach USD 22.1 billion by 2032 at 15% CAGR.
EU GMP Annex 1 revisions accelerated investment in advanced aseptic technologies.
Germany, Switzerland, and Ireland remain major hubs for sterile manufacturing.
Key Players:
Catalent, Inc || Thermo Fisher Scientific Inc. || Recipharm AB || Jubilant HollisterStier || Grand River Aseptic Manufacturing || Afton Scientific Lonza || Catalent || Baxter BioPharma Solutions || Siegfried || Vetter Pharma || Recipharm || Thermo Fisher || Alcami || PCI Pharma || B Braun OEM
Key Highlights
1. Lonza expanded sterile fill–finish capacity in Switzerland with a USD 400M investment.
2. Catalent reported a 22% YoY increase in biologics injectable manufacturing revenue.
3. Vetter Pharma launched next-gen automated syringe filling technology.
4. Thermo Fisher completed three facility expansions across the U.S. and Italy.
Recent Developments
Catalent inaugurated a high-speed prefilled syringe line in Indiana (2025).
Lonza partnered with multiple biotech companies for mAb fill–finish programs (2024).
Siegfried acquired two EU aseptic facilities to expand the injectable portfolio (2025).
Recipharm launched an advanced Annex 1-compliant isolator platform (2024).
Buy This Report with Year-End Offer (Buy 1 report: Get 30% OFF | Buy 2 reports: Get 50% OFF each! Limited time offer): https://www.datamintelligence.com/buy-now-page?report=north-america-and-europe-sterile-injectables-cmo-market
Conclusion:
The North America and Europe Sterile Injectables CMO market is set for strong, sustained growth as demand for biologics, oncology therapies, and advanced injectable formulations continues to rise. With major capacity expansions, strict regulatory alignment, and increasing outsourcing by pharma and biotech companies, the region remains a global hub for sterile manufacturing excellence.
Market Outlook
Fill–finish services to exceed USD 32 billion by 2032.
Prefilled syringes to capture 28% of the market by 2032.
Biologics-focused sterile injectables to grow at 17% CAGR.
Related Reports:
Sterile Injectables CMO Market Rising at 13.1% CAGR - DataM Intelligence @ https://www.datamintelligence.com/research-report/sterile-injectables-cmo-market
Sterile Injectables Market to Reach $1.07B by 2033 - DataM Intelligence @ https://www.datamintelligence.com/research-report/sterile-injectable-market
Sai Kiran
DataM Intelligence 4market Research LLP
877-441-4866
sai.k@datamintelligence.com
Visit us on social media:
LinkedIn
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.