Pegfilgrastim Biosimilar Market to Reach USD 9.3 Billion by 2035 as Biologics Transformation Accelerates in Oncology
Key Players in the Pegfilgrastim Biosimilar Market: Viatris Inc./Biocon Biologics Ltd., Sandoz International GmbH, Coherus BioSciences Inc.,
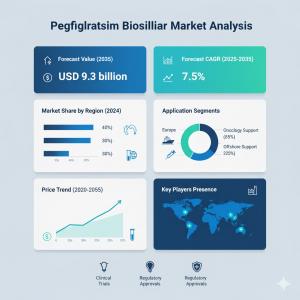
ROCKVILLE, MD, UNITED STATES, October 24, 2025 /EINPresswire.com/ -- The global pegfilgrastim biosimilar market is on a remarkable growth trajectory, driven by rising adoption of cost-effective biologic alternatives, expanding cancer prevalence, and healthcare systems’ shift toward value-based treatments.According to a recent report by Fact.MR, the market is valued at USD 4.5 billion in 2025 and is projected to reach USD 9.3 billion by 2035, registering an impressive CAGR of 7.5% over the forecast period. This represents an absolute increase of USD 4.8 billion, underscoring the biosimilars’ critical role in reducing treatment costs and improving access to life-saving oncology therapies.
Strategic Market Drivers
Rising Global Cancer Incidence and Treatment Demand
The increasing prevalence of cancer worldwide is amplifying the need for effective supportive therapies to manage chemotherapy-induced neutropenia. Pegfilgrastim, a long-acting granulocyte colony-stimulating factor (G-CSF), plays a crucial role in maintaining optimal white blood cell counts during chemotherapy. As oncology treatment volumes grow, biosimilar alternatives are gaining traction for their comparable efficacy and affordability, driving market penetration across both developed and emerging healthcare systems.
Expiry of Patents and Entry of Cost-competitive Biosimilars
The expiration of patents for originator biologics such as Neulasta® has created substantial opportunities for biosimilar manufacturers. Multiple approvals from regulatory agencies like the U.S. FDA and the European Medicines Agency (EMA) have accelerated market entry, enabling broader access and intensifying competition. This has not only improved price accessibility but also encouraged pharmaceutical companies to invest in advanced manufacturing capabilities for high-quality biosimilars.
Healthcare Cost Optimization and Policy Support
Global healthcare systems are increasingly embracing biosimilars as a key lever to reduce expenditure without compromising clinical outcomes. Reimbursement incentives, physician education programs, and government-backed biosimilar adoption initiatives in regions such as North America, Europe, and Asia-Pacific are contributing to rapid market growth.
Advancements in Biologic Manufacturing and Distribution
Technological innovation in biologics production—such as improved protein expression systems, optimized purification processes, and cold-chain logistics—has enhanced biosimilar scalability and quality assurance. Companies are increasingly adopting continuous manufacturing and digital monitoring technologies to ensure consistent product efficacy and regulatory compliance.
Regional Growth Highlights
North America: Market Leadership through Regulatory Approvals and Uptake
The U.S. dominates the global pegfilgrastim biosimilar landscape due to strong oncology demand, favorable reimbursement policies, and a robust pipeline of FDA-approved biosimilars. Canada is also witnessing growing acceptance, supported by healthcare cost-control measures and patient awareness programs.
Europe: Established Biosimilar Ecosystem and Cost-driven Expansion
Europe remains a key growth hub owing to a mature biosimilar framework, physician acceptance, and proactive government policies promoting substitution. Markets such as Germany, the United Kingdom, and France lead adoption, supported by competitive pricing and widespread healthcare integration.
East Asia: Rapid Market Development and Local Manufacturing Strength
East Asia, particularly Japan, South Korea, and China, is emerging as a pivotal region for biosimilar production and consumption. Local pharmaceutical companies are investing heavily in R&D and clinical trials, leveraging strong manufacturing infrastructure and regulatory alignment to expand exports.
Emerging Markets: Expanding Access and Affordability
Latin America, the Middle East, and parts of Africa are gradually integrating biosimilars into national health systems. Government initiatives to improve oncology treatment access, coupled with partnerships with global biologics manufacturers, are fueling steady regional adoption.
Market Segmentation Insights
By Distribution Channel
Hospital Pharmacies: Leading segment due to high chemotherapy administration volumes and institutional purchasing agreements.
Retail Pharmacies: Expected to grow as outpatient oncology treatments and home-based care models expand.
Online Pharmacies: Witnessing growth with the digitalization of healthcare and e-prescription trends.
By Indication
Chemotherapy-induced Neutropenia: Dominant indication segment, representing the largest market share.
Bone Marrow Transplant Support: Emerging segment supported by expanding hematologic oncology treatments.
Challenges and Market Considerations
Despite promising prospects, the pegfilgrastim biosimilar market faces challenges:
Complex Regulatory Pathways: Stringent analytical and clinical comparability requirements delay product launches.
Physician Hesitancy: Limited education and entrenched trust in reference biologics hinder rapid substitution in certain markets.
Manufacturing Complexity: Maintaining biologic consistency across batches demands significant investment in advanced bioprocessing.
Pricing Pressure: Intense competition and cost-containment measures can erode profit margins, necessitating operational efficiency.
Competitive Landscape
The pegfilgrastim biosimilar market is highly consolidated, with leading biopharmaceutical companies focusing on innovation, regulatory approvals, and regional partnerships.
Key Players in the Pegfilgrastim Biosimilar Market
Viatris Inc./Biocon Biologics Ltd.
Sandoz International GmbH
Coherus BioSciences Inc.
Fresenius Kabi AG
Pfizer Inc.
Teva Pharmaceutical Industries Ltd.
Celltrion Inc.
Samsung Bioepis Co. Ltd.
Mundipharma International Ltd.
These companies are advancing competitive differentiation through superior biologic engineering, patient access programs, and co-development partnerships with oncology centers.
Maufracture’s Strategic Positioning
Maufracture is strategically poised to strengthen its presence in the global biosimilar space through:
Innovative Biologic Development: Investing in next-generation biosimilars and long-acting G-CSF formulations to improve therapeutic outcomes.
Global Market Expansion: Targeting regulatory approvals in the U.S., EU, and Asia-Pacific to enhance accessibility and coverage.
Sustainable Manufacturing: Implementing eco-efficient production methods and digitalized quality control for biologics.
Collaborative Partnerships: Working closely with healthcare institutions and oncology networks to expand patient reach and clinical integration.
Future Outlook: Toward Affordable Biologic Therapies
The coming decade marks a transformative phase for the pegfilgrastim biosimilar market, as the world transitions toward cost-efficient, high-efficacy biologic treatments. Increasing acceptance among healthcare providers, coupled with manufacturing innovation and regulatory harmonization, will continue to propel global adoption.
Manufacturers that blend scientific rigor with accessibility and sustainability will emerge as key enablers of the next generation of cancer care. With robust innovation pipelines and strategic global alliances, Maufracture stands ready to advance biosimilar excellence—delivering reliable, affordable, and life-improving therapies to patients worldwide.
Request for Discount: https://www.factmr.com/connectus/sample?flag=S&rep_id=1494
Buy Now at USD 2900: https://www.factmr.com/checkout/1494
Check out More Related Studies Published by Fact.MR Research:
Editor’s Note:
The information in this press release is sourced from the comprehensive market study conducted by Fact.MR on the Pegfilgrastim Biosimilar Market. The report provides detailed insights into market trends, growth drivers, regional dynamics, and competitive strategies shaping the global biosimilar landscape.
S. N. Jha
Fact.MR
+1 628-251-1583
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.