Japan Software as a Medical Device (SaMD) Market to Hit US$ 96.2 Million by 2033, Growing at 17.3% CAGR
AI-powered diagnostics, cloud-based healthcare, and IoT-driven patient monitoring are transforming Japan’s Software as a Medical Device landscape.
𝗚𝗲𝘁 𝗮 𝗦𝗮𝗺𝗽𝗹𝗲 𝗣𝗗𝗙 𝗕𝗿𝗼𝗰𝗵𝘂𝗿𝗲 𝗼𝗳 𝘁𝗵𝗲 𝗥𝗲𝗽𝗼𝗿𝘁 (𝗨𝘀𝗲 𝗖𝗼𝗿𝗽𝗼𝗿𝗮𝘁𝗲 𝗘𝗺𝗮𝗶𝗹 𝗜𝗗 𝗳𝗼𝗿 𝗮 𝗤𝘂𝗶𝗰𝗸 𝗥𝗲𝘀𝗽𝗼𝗻𝘀𝗲):
https://www.datamintelligence.com/download-sample/japan-software-as-a-medical-device-market
SaMD operates independently of hardware and leverages mobile devices and cloud platforms, offering scalable, accessible solutions for both healthcare professionals and patients. The diagnostics segment, supported by AI-powered imaging and analytics, has emerged as the leading application due to demand for early disease detection and growing chronic disease prevalence. Urban regions, with higher hospital density and robust digital infrastructure, lead the market in adoption due to their advanced healthcare ecosystems and openness to innovation.
Key Highlights from the Report
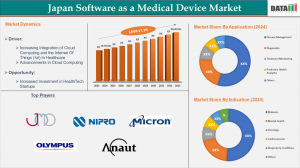
➤ Japan Software as a Medical Device market reached US$ 19.55 million in 2024, set to surpass US$ 96.2 million by 2033.
➤ Rapid market expansion is fueled by AI-driven diagnostics and cloud-based healthcare analytics.
➤ Diagnostics remains the dominant application segment, ensuring early and precision disease detection.
➤ Leading players include Japan Medical Device Corporation, Nipro Corporation, Micron, Inc., Olympus Medical Systems, and Anaut Inc.
➤ Strategic partnerships and regulatory approvals, such as Monitor Corporation and Doctor-NET, are accelerating product adoption.
➤ The market faces digital literacy barriers among the elderly and traditional healthcare professionals.
Market Segmentation
By Application
Digital health solutions are categorized into Disease Management, Diagnostics, Treatment Monitoring, Predictive Health Analytics, and Others. Disease management and treatment monitoring dominate the market, driven by rising adoption of connected devices and real-time patient data tracking.
By Indication
Based on indication, the market covers Diabetes, Mental Health, Oncology, Cardiovascular, Respiratory Conditions, and Others. Diabetes and cardiovascular conditions lead due to the growing use of remote monitoring tools, while mental health and oncology applications are rapidly expanding with AI-driven health insights.
Looking For A Detailed Full Report? Get it here:
https://www.datamintelligence.com/buy-now-page?report=japan-software-as-a-medical-device-market
Regional Insights
Japan’s SaMD market growth is notably concentrated in metropolitan hubs like Tokyo, Osaka, and Nagoya, where advanced healthcare infrastructure drives early adoption. Urban hospitals, research centers, and tertiary care institutions leverage cloud-based diagnostic platforms and remote patient monitoring to mitigate resource gaps and enhance efficiency. The central region leads the market due to proactive digital health policies, strong regulatory support, and high penetration of connected devices.
Rural and less-digitized regions lag in adoption due to limited connectivity and training for older healthcare professionals. However, government initiatives are underway to extend telemedicine networks, introduce cloud-based health records, and incentivize digital literacy among practitioners in these areas. A gradual convergence of rural-urban market growth is expected as infrastructure and policy frameworks mature.
Market Dynamics
Market Drivers
Japan’s Software as a Medical Device market is propelled by the increased integration of cloud computing and IoT in healthcare delivery. Cloud-based solutions enable secure, scalable data storage and real-time analytics, empowering clinicians to make informed, patient-centric decisions. The proliferation of IoT devices allows remote monitoring, telehealth expansion, and early disease detection, reducing hospital visits and enhancing patient outcomes. Demand for cost-effective healthcare and regulatory approvals for AI-based diagnostics further accelerate market growth.
Market Restraints
Despite rapid advances, market expansion is held back by limited digital literacy among Japan’s aging population and some healthcare professionals. Many elderly patients find digital health platforms challenging, slowing SaMD adoption for remote monitoring and AI diagnostics. Traditional practitioners may be resistant to workflow changes, hindering digital integration and reducing trust in software-based interventions. These challenges highlight the need for targeted training programs and intuitive software design to foster confidence in SaMD solutions.
Market Opportunities
There is significant potential for new entrants and established players to capitalize on precision diagnostics, cloud-based care coordination, and telehealth platforms. Partnerships between Japanese firms and global digital health leaders, exemplified by collaborations like Monitor Corporation and Doctor-NET, are expanding the reach of AI-powered diagnostic products. Regulatory reforms supporting digital therapeutics, predictive analytics, and value-based pricing create new avenues for product launches tailored to Japan’s healthcare needs. As post-market surveillance and real-world evidence tools advance, companies can enhance safety, drive continuous improvement, and support patient-centric innovations.
Get Customization in the report as per your requirements:
https://www.datamintelligence.com/customize/japan-software-as-a-medical-device-market
Reasons to Buy the Report
✔ Comprehensive coverage of current and emerging market trends.
✔ Analysis of regulatory changes, industry challenges, and future opportunities.
✔ Detailed segmentation by application, indication, and leading end-users.
✔ Competitive landscape review with key player strategies.
✔ Insight into product performance, market positioning, and forecast growth.
Frequently Asked Questions (FAQs)
◆ How Big is the Japan Software as a Medical Device Market in 2024?
◆ What is the Projected Growth Rate of the Japan SaMD Market?
◆ Who are the Key Players in the Japan Software as a Medical Device Market?
◆ Which Application Segment Leads the Japan SaMD Industry?
◆ What are the Major Drivers Influencing the Japan SaMD Market?
Company Insights
• Japan Medical Device Corporation
• Nipro Corporation
• Micron, Inc.
•Olympus Medical Systems
• Anaut Inc.
Recent developments:
✅ In October 2025, Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) reiterated its target review period of six months for priority-pathway SaMD products, as outlined in its mid-term plan covering FY2024–2028, strengthening its stance on accelerating SaMD approval.
✅ In August 2025, the Ministry of Health, Labour and Welfare (MHLW) published updated “Guidelines of Criteria for Judging SaMD” clarifying that software delivering individual-specific disease diagnosis or risk assessments is likely regulated as a medical device under the Pharmaceuticals and Medical Devices Act (PMD Act).
✅ In September 2025, the medical-device pricing body in Japan proposed developing clearer reimbursement criteria for SaMDs, signalling that software is increasingly treated akin to traditional devices in pricing and evaluation frameworks.
Conclusion
The Japan Software as a Medical Device market stands at the forefront of digital transformation in healthcare, driven by AI, IoT, and strategic collaborations among leading providers. While digital literacy and workflow integration pose ongoing challenges, proactive regulations and partnerships continue to unlock new markets for SaMD technologies, ensuring improved diagnostics, enhanced clinical decisions, and more accessible care for all. As technology evolves and precision medicine gains momentum, stakeholders in the Japanese SaMD ecosystem can expect dynamic growth, diversified opportunities, and remarkable advancements in patient health outcomes.
Sai Kiran
DataM Intelligence 4market Research LLP
877-441-4866
sai.k@datamintelligence.com
Visit us on social media:
LinkedIn
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.