$4.1+ Bn Ventricular Assist Devices Market growing at a CAGR of 8.1% to 2034
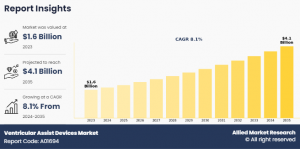
PORTLAND, OREGON, UNITED STATES, June 14, 2024 /EINPresswire.com/ -- The ventricular assist devices (VADs) market, valued at $1.6 billion in 2023, is anticipated to surge to $4.1 billion by 2035, reflecting a compound annual growth rate (CAGR) of 8.1% from 2024 to 2035. The primary driver of this growth is the increasing incidence of heart failure. According to the Centers for Disease Control and Prevention (CDC), as of January 2023, heart disease remains the leading cause of death among both men and women in the U.S., with approximately 6.2 million adults affected by heart failure. This high prevalence significantly boosts the demand for VADs, thereby propelling market expansion.
𝐆𝐞𝐭 𝐚 𝐒𝐚𝐦𝐩𝐥𝐞 𝐂𝐨𝐩𝐲 𝐨𝐟 𝐭𝐡𝐢𝐬 𝐑𝐞𝐩𝐨𝐫𝐭: https://www.alliedmarketresearch.com/request-sample/2024
Market Segmentation
By Product
The market is segmented into:
• Left Ventricular Assist Devices (LVADs)
• Right Ventricular Assist Devices (RVADs)
• Biventricular Assist Devices (BIVADs)
In 2023, LVADs held the largest market share due to their widespread use in supporting the heart's primary pumping chamber. The high prevalence of left ventricular dysfunction in heart failure cases further underpins the dominance of LVADs.
By Application
The application-based segments include:
• Bridge-to-Transplant (BTT)
• Destination Therapy
• Bridge-to-Recovery (BTR)
• Bridge to Candidacy Therapy
BTT accounted for the largest market share in 2023, driven by its effectiveness in enhancing transplant success rates. Destination therapy, however, is projected to witness the fastest growth rate, fueled by the recognition of VADs as a long-term treatment for patients ineligible for heart transplants.
By Design
The design-based segmentation is:
• Transcutaneous Ventricular Assist Devices
• Implantable Ventricular Assist Devices
Implantable VADs dominated the market in 2023, owing to ongoing technological advancements and intensive research efforts aimed at improving device efficiency and reliability.
By Age
Age-based segmentation includes:
• Adults
• Pediatrics
The adult segment captured the largest market share in 2023 due to the high incidence of heart failure in adults. However, the pediatric segment is expected to grow rapidly, driven by advancements in pediatric VAD technology.
By Region
Regionally, the market is analyzed across:
• North America (U.S., Canada, Mexico)
• Europe (Germany, France, UK, Italy, Spain, Rest of Europe)
• Asia-Pacific (China, Japan, India, Australia, South Korea, Rest of Asia-Pacific)
• LAMEA (Brazil, South Africa, North Africa, Rest of Middle East & Africa)
North America led the market in 2023, supported by a robust healthcare infrastructure, significant healthcare expenditure, advanced therapeutic technology adoption, strong R&D activities, and supportive government policies.
Competitive Landscape
Key players in the market, such as Abbott Laboratories and LivaNova, leverage strategies like product launches, acquisitions, and approvals to strengthen their market position. Notably, in September 2022, Abiomed received two FDA approvals for clinical research on Impella heart pumps for patients with acute myocardial infarction (AMI) cardiogenic shock, highlighting the competitive and innovative nature of the market.
This detailed segmentation and analysis reflect the diverse and dynamic nature of the ventricular assist devices market, underscoring the significant advancements and growing demand in the field of cardiac care.
Key Market Players
1. Fineheart
2. Carmat SA
3. AdjuCor GmbH
4. LivaNova PLC
5. Evaheart
6. Abbott Laboratories
7. Abiomed
8. Berlin Heart GmbH
9. CH Biomedical
10. Bivacor Inc.
𝐄𝐧𝐪𝐮𝐢𝐫𝐞 𝐁𝐞𝐟𝐨𝐫𝐞 𝐁𝐮𝐲𝐢𝐧𝐠: https://www.alliedmarketresearch.com/purchase-enquiry/2024
Key Benefits for Stakeholders
1. Comprehensive Market Analysis:
• The report provides a detailed quantitative analysis of various market segments, current trends, future estimations, and market dynamics from 2023 to 2035. This analysis helps identify key opportunities within the ventricular assist devices (VADs) market.
2. Insight into Market Drivers and Restraints:
• It offers extensive information on key drivers, restraints, and opportunities impacting the market. This enables stakeholders to understand the underlying factors influencing market growth and develop strategies accordingly.
3. Strategic Decision-Making:
• Porter's Five Forces Analysis is included to highlight the power dynamics between buyers and suppliers. This helps stakeholders make informed, profit-oriented business decisions and fortify their supplier-buyer relationships.
4. In-Depth Segmentation Analysis:
• The report includes a thorough analysis of market segmentation by product, application, design, age, and region. This assists stakeholders in pinpointing the most lucrative market opportunities.
5. Regional Revenue Insights:
• Major countries in each region are mapped according to their revenue contributions to the global market. This geographic analysis helps stakeholders identify high-revenue regions and tailor their strategies to these markets.
6. Market Player Positioning:
• The report features a detailed market player positioning analysis, facilitating benchmarking and offering a clear understanding of the competitive landscape. This helps stakeholders assess their position relative to key competitors and identify areas for improvement.
7. Trend Analysis and Growth Strategies:
• An analysis of both regional and global trends is included, along with insights into key players, market segments, application areas, and market growth strategies. This comprehensive overview helps stakeholders stay informed about market developments and devise effective growth strategies.
By leveraging these insights, stakeholders can make well-informed decisions, optimize their strategies, and capitalize on emerging opportunities within the ventricular assist devices market.
David Correa
Allied Market Research
+1 800-792-5285
email us here
Visit us on social media:
Facebook
Twitter
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.