Senex Publication Reveals the Importance of CDK8/19 Inhibition as Treatment for Castration Resistant Prostate Cancer
The Journal of Clinical Investigation paper discusses the role of Mediator kinases CDK8/CDK19, pleiotropic regulators of transcriptional reprogramming, in CRPC
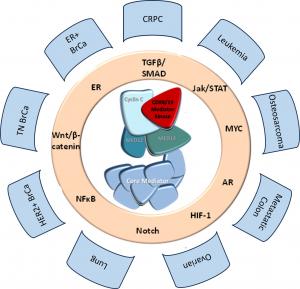
Senex Biotechnology scientists and their colleagues at the University of South Carolina and the Medical University of South Carolina have authored a manuscript recently published in the Journal of Clinical Investigation discussing the role of Mediator kinases CDK8 and CDK19, pleiotropic regulators of transcriptional reprogramming, in castration resistant prostate cancer (CRPC). (Mediator kinase inhibition reverses castration resistance of advanced prostate cancer, J Clin Invest. 2024. https://doi.org/10.1172/JCI176709). Their research demonstrates that genetic or pharmacological inhibition of CDK8 and CDK19 reverses the castration-resistant phenotype and restores the sensitivity of CRPC xenografts to androgen deprivation in vivo. The data presented in the publication indicate that Mediator kinases mediate androgen-independent in vivo growth of CRPC, supporting the development of the Company’s CDK8/19 inhibitors for the treatment of this presently incurable disease.
“This paper covers many years of painstaking research that combined bioinformatic analysis of clinical prostate cancers, genetic modifications of prostate cancer cells, and a very extensive transcriptomic analysis of tumor models, with in vitro and in vivo characterization of the CDK8/19 inhibitor that Senex is now developing for the clinic. I hope that patients with the presently incurable advanced prostate cancer will be able to benefit from this new type of drug that has emerged from our studies” - Mengqian Chen, Ph.D., Director of Research at Senex Biotechnology and senior author of the article.
“Senex believes that SNX631-6, the Company’s clinical candidate, is the best-in-class CDK8/19 inhibitor, with low nanomolar potency, outstanding target specificity and excellent oral bioavailability. These attributes contribute to the excellent safety profile seen in IND-enabling toxicology studies. Senex plans to initiate first in human studies in 2025"- Dennis I. Goldberg, Ph.D., Chief Executive Officer at Senex Biotechnology.
"I am very excited about the strong in vivo efficacy of CDK8/19 inhibitors in advanced prostate cancer discovered by my colleagues, and the congruent effects of the genetic and pharmacological inhibition of Mediator kinases, which highlight the pertinence of this treatment to the etiology of prostate cancer. Once Senex’s drug reaches the clinic, I will not hesitate to take it if I ever need it for myself”- Igor B. Roninson, Ph.D., Founder and Chief Science Officer at Senex Biotechnology.
“Castration-resistant prostate cancers are more resilient to different therapies than the less advanced castration-responsive cancers. It is very promising that CDK8/19 inhibitors, uniquely, become more potent when prostate cancers become castration resistant. Once such cancers stop responding to the latest anti-androgens, the principal choices offered to the patient are the toxic chemotherapy and radioactive conjugates. It would be very appealing for the patient to have a CDK8/19 inhibitor as an alternative option that is not radioactive and that we expect not to be toxic” - George Wilding, M.D., Chief Medical Officer at Senex Biotechnology.
About Senex Biotechnology
Senex Biotechnology is a drug discovery and development company focused on cancer therapeutics. Senex’s lead program targets CDK8/19, a protein that regulates gene expression and is required by cancer cells to adapt to adversarial conditions; such adaptation leads to cancer drug resistance and metastasis. Senex is developing highly selective small-molecule inhibitors of CDK8/19 for the treatment of presently incurable types of prostate cancer, breast cancer, osteosarcoma and leukemia. We are also investigating the utility of these inhibitors for different cancers in combination with other therapeutics, as well as for inflammation, cardiovascular and other diseases. Our latest, highly potent and selective drug candidate is anticipated to enter clinical trials in 2025.
Senex was founded by Dr. Igor Roninson, based on the discovery in his academic laboratory of a novel biological pathway associated with aging (senescence) and involved in cancer and other chronic diseases, as well as the use of functional genomics technologies to identify novel drug targets that are required by tumor cell but not by normal tissues. Senex has won 16 competitive grant awards from the National Institutes of Health, Department of Defense Congressionally Directed Medical Research Programs (DoD CDMRP) and the Alzheimer’s Drug Discovery Foundation. The Company’s work on prostate cancer drug development was supported by a Phase II Small Business Innovation Research (SBIR) grant from the National Cancer Institute and a Prostate Cancer Research Program Translational Science Award from the DoD CDMRP.
For further information, visit www.senexbio.com.
Contact information:
Dennis I Goldberg
Senex Biotechnology, Inc.
+1 508-878-7589
email us here
Visit us on social media:
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.