Biopharmaceutical CMO Market Trends, Size, Growth, Opportunities, Challenges, and Forecast 2030
Biopharmaceutical CMO Market Soars to USD 79.77 Billion by 2030, Driven by Expanding Biologics Pipeline
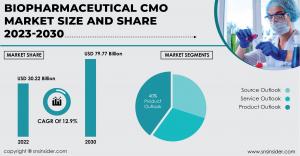
AUSTIN, TEXAS, UNITED STATES, March 14, 2024 /EINPresswire.com/ -- The SNS Insider report reveals that the Biopharmaceutical CMO Market is expected to reach 79.77 billion by 2030, reflecting a compound annual growth rate (CAGR) of 12.9% from its estimated value of USD 30.22 billion in 2022.The biopharmaceutical Contract Manufacturing Organization (CMO) market report provides a comprehensive analysis of the current trends, key drivers, challenges, and growth opportunities in the industry. It offers insights into the competitive landscape, regulatory framework, and technological advancements shaping the market. The report also covers market dynamics such as mergers and acquisitions, partnerships, and collaborations among key players. Furthermore, it highlights the increasing demand for outsourcing services by biopharmaceutical companies to reduce costs, improve efficiency, and accelerate drug development processes. With a focus on quality assurance and compliance with stringent regulatory guidelines, CMOs are playing a vital role in supporting the manufacturing capabilities of pharmaceutical companies worldwide. Overall, the biopharmaceutical CMO market report serves as an essential tool for stakeholders to make informed decisions regarding investment strategies and business expansion opportunities in this rapidly evolving sector.
Market Report Scope:
Biopharmaceutical Contract Manufacturing Organizations (CMOs) play a pivotal role in the pharmaceutical sector, offering a spectrum of services from drug manufacturing to packaging. This outsourcing trend allows major players to concentrate on core activities such as drug discovery and marketing. Biopharmaceutical CMOs handle critical processes like cell culture, fermentation, purification, formulation, and fill-finish, ensuring compliance with stringent regulatory standards like Good Manufacturing Practices (GMP).
Get PDF Sample Copy of Report: https://www.snsinsider.com/sample-request/2938
Major Key Players in the Biopharmaceutical CMO Market:
• Lonza
• Thermo Fisher Scientific
• Cambrex Corporation
• Catalent
• Fujifilm
• Siegfried Holding Ag
• Samsung Biologics
• AbbVie
• Binex
• Parexel international Corporation
Market Analysis
The burgeoning pipeline of biologics is a key driver for market growth. Many drug manufacturers now outsource to CMOs, leveraging their cost-effective mass production capabilities and avoiding substantial capital investments. This trend offers substantial growth opportunities in the biopharmaceutical CMO sector. The increased demand for biopharmaceuticals, driven by novel drug projects and ongoing growth in biopharmaceuticals, fuels the expansion of CMO capabilities. Contract manufacturing organizations are adapting to meet the rising requirements, expanding both their capabilities and capacities.
Key Segments Covered in Biopharmaceutical CMO Market Report:
Source Outlook
• Mammalian
• Non- Mammalian
Service Outlook
• Contract Manufacturing
• Contact Research
Product Outlook
• Biologics
• Biosimilars
The mammalian segment dominates with over 50% market share due to its ability to provide human-like post-translational modifications to complex protein therapeutics. The introduction of enhanced expression systems, process monitoring solutions, cell line engineering tools, automated screening methods, and disposable devices further strengthens its position. The non-mammalian segment is witnessing rapid growth, particularly microbial cell lines like Yeast Saccharomyces cerevisiae, contributing significantly to revenue.
Make Enquiry About Biopharmaceutical CMO Market Report: https://www.snsinsider.com/enquiry/2938
Impact of Economic Slowdown
The impact of an economic slowdown on the biopharmaceutical Contract Manufacturing Organization (CMO) market can be significant. During times of economic downturn, pharmaceutical companies may delay or cut back on their research and development budgets, leading to decreased demand for CMO services. This can result in lower contract volumes and reduced revenues for CMOs, forcing them to adjust their business operations accordingly. Additionally, economic uncertainty may lead to reduced investor confidence in the biopharmaceutical industry as a whole, resulting in decreased funding for new drug development projects and ultimately affecting the demand for CMO services. In order to navigate through such challenging times, CMOs must focus on cost optimization strategies, diversifying their service offerings, and forming strategic partnerships with pharmaceutical companies to ensure long-term sustainability in the market.
Impact of Russia-Ukraine War
The ongoing Russia-Ukraine war has had a significant impact on the biopharmaceutical Contract Manufacturing Organization (CMO) market, as both countries are major players in the pharmaceutical industry. The conflict has disrupted supply chains and manufacturing capabilities in the region, leading to delays in production and distribution of key drugs and vaccines. Many CMOs have been forced to find alternative suppliers and reorganize their operational strategies to mitigate the effects of the war. Additionally, political instability and uncertainty surrounding the conflict have led to increased costs for CMOs operating in the region, as well as hindered collaboration between companies. Overall, the Russia-Ukraine war has created challenges for the biopharmaceutical CMO market, with potential long-term implications on global pharmaceutical supply chains and production capabilities.
Regional Developments:
Dominating the market, North America benefits from a developed healthcare system, a robust biopharmaceutical industry, and a favorable regulatory environment. The concentration of biopharmaceutical businesses and research facilities, coupled with technological advancements, positions the region as a leader.
Europe secures the second-largest share, driven by established pharmaceutical industries in countries like Switzerland, Germany, and the United Kingdom. A strong regulatory environment and significant investment in academic research facilities contribute to its market presence.
With the highest CAGR, the APAC region is propelled by a large patient population, reduced production costs, improved regulatory frameworks, and government support. The region offers opportunities for CMOs specializing in generic biologics and biosimilars.
Key Takeaways:
• Biologics Pipeline Driving Growth: The thriving biologics pipeline remains a pivotal force, steering the Biopharmaceutical CMO Market to unprecedented heights.
• Global Dominance of North America: North America's established healthcare system and technological advancements position it as a global leader in the biopharmaceutical CMO market.
• APAC's Rapid Growth: The APAC region, fueled by factors like a vast patient population and government support, exhibits the highest CAGR in the biopharmaceutical CMO market.
Recent Developments:
• April 2021: Thermo Fisher Scientific announced the acquisition of PPD, Inc., enhancing its market position by expanding production capability and customer base.
• May 2021: Samsung Biologics and Moderna Inc. signed an agreement for fill & finish services for Moderna's COVID-19 Vaccine, highlighting strategic collaborations within the industry.
Buy Biopharmaceutical CMO Market Report: https://www.snsinsider.com/checkout/2938
Table of Content
Chapter 1 Introduction
Chapter 2 Research Methodology
Chapter 3 Biopharmaceutical CMO Market Dynamics
Chapter 4 Impact Analysis (COVID-19, Ukraine- Russia war, Ongoing Recession on Major Economies)
Chapter 5 Value Chain Analysis
Chapter 6 Porter’s 5 forces model
Chapter 7 PEST Analysis
Chapter 8 Biopharmaceutical CMO Market Segmentation, By Source Outlook
Chapter 9 Biopharmaceutical CMO Market Segmentation, By Service Outlook
Chapter 10 Biopharmaceutical CMO Market Segmentation, By Product Outlook
Chapter 12 Regional Analysis
Chapter 13 Company profile
Chapter 14 Competitive Landscape
Chapter 15 Use Case and Best Practices
Chapter 16 Conclusion
Akash Anand
SNS Insider Pvt. Ltd
+1 415-230-0044
email us here
Visit us on social media:
Facebook
Twitter
LinkedIn
Instagram
YouTube
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.