3G Microbial Cell Factories: Achieving Sustainable Goals with Engineered Microorganisms
Scientists review the progress made in development of artificial biological systems in the past decade
CHINA, December 11, 2023 /EINPresswire.com/ -- Third generation biorefineries aim to utilize microorganisms and microbial enzyme systems driven by natural energy sources of light and electricity to fix one-carbon (C1) sources. Artificial biological systems fix C1 sources to generate green energy along with beneficial by-products. In a recent study, scientists from China review the developments made in the development of various engineered biological systems with special emphasis on artificial autotrophs, tandem enzymatic systems, and chemo-hybrid systems.
Fossil fuels are at the heart of most human activities, contributing to the increase in greenhouse gas emissions and the ever-rising atmospheric carbon dioxide (CO2) levels. CO2 levels are projected to increase exponentially in future, resulting in severe environmental and ecological impacts. A beacon of hope amidst this chaos are eco-friendly alternatives to fossil fuels.
Green energy sources can be developed using advanced biotechnological techniques. One such intervention is the use of biorefineries or microbial cell factories, that convert biomass (organic matter like plants and solid waste) into energy and valuable by-products. The first (1G) and second (2G) generation biorefineries produce biofuels and valuable chemicals from sugar-based feedstocks and biomass, respectively. The third generation (3G) biorefineries are now becoming increasingly popular and aim to produce beneficial products from one-carbon (C1) sources like CO2, formaldehyde (HCHO), formic acid (HCOOH), methane (CH4), and methanol (CH3OH). Why C1 sources? C1 sources produce sustainable energy from chemical bond breakdown, electricity, and/or natural light.
In a new study, Prof. Yajie Wang from Westlake University, China, along with her colleagues Wei Zhong and Hailong Li, reviewed the progress made in the development of artificial biological systems for 3G refineries over the last decade. Their findings were published on 31 October 2023 in Volume 5 of BioDesign Research. Prof. Wang remarks, “This promising technology represents an important step toward sustainable development, and it can help address some of modern society's most pressing environmental challenges. However, to compete with the petroleum industry, it is critical to identify the most viable pathways for C1 utilization, as well as the productivity and yield of the target products.”
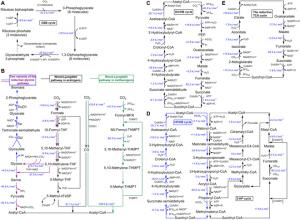
The authors discussed three important artificial systems utilizing C1, namely, artificial autotrophic organisms, tandem enzymatic systems, and chemo-bio hybrid systems. Such systems are either reconstructed based on the natural system or created from scratch.
Microorganisms are mostly heterotrophs, i.e. they depend on external sources for food and energy. However, with the advancement of biotechnology, heterotrophs can now be engineered to chemoautotrophs, that derive energy from the breakdown of chemical substances like C1. Several microbes, including bacteria and yeasts like E. coli, P. pastoris, S. cerevisiae, and Candida boidinii have been modified to produce enzymes and cofactors of natural C1 utilization pathways, they do not otherwise possess. However, energy imbalance, oxygen sensitivity, and poor enzyme activities have been major limitations in the development of C1 utilizing efficient chemoautotrophs.
Tandem enzymatic systems combine multiple enzymes that function together for C1 fixation. Various scientists have independently developed many artificial C1 fixation pathways in different microorganisms. One of the most noteworthy developments is the CETCH (crotonyl-CoA/ethylmalonyl-CoA/hydroxybutyryl-CoA) pathway that has outperformed chemical as well as natural fixation pathways. Although theoretically these systems claim to maximize yield, there are limitations such as the requirement of purified enzymes and well-controlled reaction conditions.
Chemo-bio hybrid systems combine biocatalysts like microbial enzymes or whole cells with electrocatalytic and/or photocatalytic systems to synthesize value-added compounds from CO2. These systems have several advantages over the conventional enzymatic process such as use of renewable energy, high-energy conversion efficiency, and wide range of biocatalysts with varied selectivity and specificity. Depending on the combination of systems, there are various types of hybrids that fix C1- electroenzymatic, electromicrobial, photoenzymatic, and photomicrobial systems.
While foreseeing a very promising future of artificial biological systems, Prof. Yajie remarks enthusiastically, “Creating an artificial biological system that can use C1 sources is an impactful research attempt because of their abundant sources, potential for sustainable and carbon-neutral production, and lack of conflict with food security.”
To summarize, with progressive advancements in biotechnology, artificial biological systems will usher in a new era of sustainable energy.
DOI
10.34133/bdr.0021
Original Source URL
https://doi.org/10.34133/bdr.0021
Lucy Wang
BioDesign Research
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.