Spinal Implants & Surgery Devices Market worth $22.67 billion by 2030 - Exclusive Report by 360iResearch
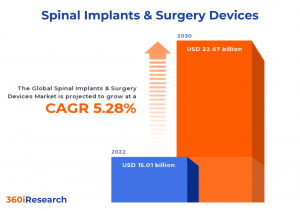
The Global Spinal Implants & Surgery Devices Market to grow from USD 15.01 billion in 2022 to USD 22.67 billion by 2030, at a CAGR of 5.28%.
PUNE, MAHARASHTRA, INDIA , November 17, 2023 /EINPresswire.com/ -- The "Spinal Implants & Surgery Devices Market by Product Type (Cervical Fusion Devices, Non-Fusion Devices, Posterior Cervical Fusion Devices), Type of Surgery (Minimally Invasive Surgeries, Open Surgeries), End-User - Global Forecast 2023-2030" report has been added to 360iResearch.com's offering.The Global Spinal Implants & Surgery Devices Market to grow from USD 15.01 billion in 2022 to USD 22.67 billion by 2030, at a CAGR of 5.28%.
Request a Free Sample Report @ https://www.360iresearch.com/library/intelligence/spinal-implants-surgery-devices?utm_source=einpresswire&utm_medium=referral&utm_campaign=sample
The spinal implants & surgery devices are used in the treatment of spinal disorders, injuries, deformities, and degenerative conditions. The scope of this market entails products such as spinal fusion devices, non-fusion devices/motion preservation devices, vertebral compression fracture (VCF) treatment devices, spinal bone stimulators, spine biologics, and minimally invasive surgical (MIS) systems. The higher prevalence of degenerative disc diseases and spine-related ailments with increasing incidences of spinal injuries due to accidents or sports activities is expanding the usage of spinal implants & surgery devices. Growing awareness about the availability and benefits associated with minimally invasive surgeries is driving the growth of the spinal implants & surgery devices market. However, high initial capital investments required for setting up manufacturing facilities and a lack of skilled surgeons with specialization in advanced spine surgeries hamper market growth. Continuous innovations in the development of technologically advanced spinal impacts & surgery devices that promote enhanced fusion rates while reducing long-term complications are expected to create opportunities for market growth.
Product Type: Growing usage of spine biologics that facilitate bone growth
Cervical fusion devices are designed to treat degenerative disc disease, instability, and deformities in the cervical spine by permanently fusing two or more vertebrae together, providing stability and relieving pain. They involve the use of plates, screws, and cages to immobilize the affected area. Non-fusion devices aim to preserve motion in the spine while providing support for spinal conditions such as spinal stenosis and degenerative disc disease. Posterior cervical fusion devices are a subcategory of cervical fusion devices used specifically for posterior surgical approaches. These systems also fuse vertebrae together using a combination of rods, screws, hooks, and wires. Spinal decompression devices are utilized in procedures such as laminectomy or discectomy to relieve pressure on the spinal cord or nerve roots. Spine biologics facilitate bone growth or regeneration in spinal surgery applications. Spine bone stimulators promote bone healing after spinal surgery by generating low-level electric fields or ultrasound waves. Thoracic fusion & lumbar fusion devices are intended for treating degenerative disc disease, instability, and deformities within the thoracic and lumbar regions of the spine. They also use various systems of screws, rods, and cages to immobilize the affected area, similar to cervical fusion devices.
End-Users: Increasing application of spinal implants & surgery devices in hospitals that reduce hospitalization time and optimize surgical outcomes
Ambulatory surgery centers are specialized healthcare facilities that offer outpatient surgical procedures in a more cost-effective and efficient setting than traditional hospital-based surgeries. They often focus on providing minimally invasive procedures, including spinal surgeries such as laminectomy, discectomy, and decompression. Ambulatory surgery centers typically prefer spinal implants and surgery devices that enable quicker patient recovery times with less postoperative pain. Hospitals, being larger healthcare facilities with diverse patient populations, have more extensive needs for spinal implants and surgery devices. They require both minimally invasive solutions for outpatient procedures and traditional spinal hardware and instrumentation for complex inpatient surgeries. These may include pedicle screw systems, rod-based constructs, expandable cages, and spinal cord stimulation devices. Ambulatory surgery centers tend to prioritize minimally invasive technologies that enable quicker recovery times, while hospitals need a broader range of solutions, including traditional hardware, for more complex cases.
Type of Surgery: Preference of spinal implants & surgery devices for minimally invasive surgeries to improve safety during spinal procedures
Minimally invasive surgeries are preferred when the objective is to reduce tissue damage, minimize scarring, and shorten recovery times. These procedures are performed using small incisions and specialized surgical instruments, which allow surgeons to access the spine without causing significant disruption to surrounding muscles and tissues. Open surgeries involve making larger incisions to provide direct access to the spine for more complicated procedures. These surgeries are chosen when extensive stabilization and fusion are required or when addressing complex spinal deformities such as scoliosis or kyphosis.
Regional Insights:
The Americas represents a highly developing landscape for the spinal implants & surgery devices market due to the increasing prevalence of degenerative spinal conditions and growing preference for minimally invasive surgeries. According to a recent study, approximately 900,000 American and 30,000 Canadian adults went under spine surgery annually. Additionally, rising advancements in technology for the development of innovative spinal implants & surgery devices are constantly creating opportunities for market growth in the Americas. The EMEA region has witnessed substantial growth in the spinal implants & surgery devices market driven by various EU countries' initiatives to support technological advancements in medical devices with constant government support. In the Middle East, countries, including the UAE, are focusing on medical tourism initiatives that attract patients seeking advanced spine treatments unavailable in their home countries, thereby fueling the market growth. Increased healthcare expenditures with a well-established healthcare system and advanced technologies are expanding the manufacturing of medical devices, including spinal implants & surgery devices, in Asia-Pacific. Further, the surge in spinal surgeries due to an increase in degenerative spine disorders attributed to a sedentary lifestyle is expanding the usage of spinal implants & surgery devices in Asia-Pacific.
FPNV Positioning Matrix:
The FPNV Positioning Matrix is essential for assessing the Spinal Implants & Surgery Devices Market. It provides a comprehensive evaluation of vendors by examining key metrics within Business Strategy and Product Satisfaction, allowing users to make informed decisions based on their specific needs. This advanced analysis then organizes these vendors into four distinct quadrants, which represent varying levels of success: Forefront (F), Pathfinder (P), Niche (N), or Vital(V).
Market Share Analysis:
The Market Share Analysis offers an insightful look at the current state of vendors in the Spinal Implants & Surgery Devices Market. By comparing vendor contributions to overall revenue, customer base, and other key metrics, we can give companies a greater understanding of their performance and what they are up against when competing for market share. The analysis also sheds light on just how competitive any given sector is about accumulation, fragmentation dominance, and amalgamation traits over the base year period studied.
Key Company Profiles:
The report delves into recent significant developments in the Spinal Implants & Surgery Devices Market, highlighting leading vendors and their innovative profiles. These include Accelus, Advin Health Care, Augmedics, B. Braun SE, Bioventus LLC, Boston Scientific Corporation, Camber Spine Technologies, LLC, Globus Medical, Inc., GPC Medical Ltd., GWS Surgicals LLP, Jayon Implants Private Limited, Johnson & Johnson Services, Inc., Kleiner Device Labs, Kuros Biosciences A.G., Medtronic PLC, NuVasive, Inc., Orthofix Medical Inc., SeaSpine Orthopedics Corporation, SI-BONE, Inc., Spineart SA, Stryker Corporation, Synapse Biomedical Inc., Vishal Surgitech Pvt. Ltd., Xtant Medical Corporate, and ZimVie Inc..
Inquire Before Buying @ https://www.360iresearch.com/library/intelligence/spinal-implants-surgery-devices?utm_source=einpresswire&utm_medium=referral&utm_campaign=inquire
Market Segmentation & Coverage:
This research report categorizes the Spinal Implants & Surgery Devices Market in order to forecast the revenues and analyze trends in each of following sub-markets:
Based on Product Type, market is studied across Cervical Fusion Devices, Non-Fusion Devices, Posterior Cervical Fusion Devices, Spinal Decompression Devices, Spine Biologics, Spine Bone Stimulators, and Thoracic Fusion & Lumbar Fusion Devices. The Thoracic Fusion & Lumbar Fusion Devices is projected to witness significant market share during forecast period.
Based on Type of Surgery, market is studied across Minimally Invasive Surgeries and Open Surgeries. The Minimally Invasive Surgeries is projected to witness significant market share during forecast period.
Based on End-User, market is studied across Ambulatory Surgery Centers and Hospitals. The Hospitals is projected to witness significant market share during forecast period.
Based on Region, market is studied across Americas, Asia-Pacific, and Europe, Middle East & Africa. The Americas is further studied across Argentina, Brazil, Canada, Mexico, and United States. The United States is further studied across California, Florida, Illinois, New York, Ohio, Pennsylvania, and Texas. The Asia-Pacific is further studied across Australia, China, India, Indonesia, Japan, Malaysia, Philippines, Singapore, South Korea, Taiwan, Thailand, and Vietnam. The Europe, Middle East & Africa is further studied across Denmark, Egypt, Finland, France, Germany, Israel, Italy, Netherlands, Nigeria, Norway, Poland, Qatar, Russia, Saudi Arabia, South Africa, Spain, Sweden, Switzerland, Turkey, United Arab Emirates, and United Kingdom. The Americas commanded largest market share of 38.34% in 2022, followed by Europe, Middle East & Africa.
Key Topics Covered:
1. Preface
2. Research Methodology
3. Executive Summary
4. Market Overview
5. Market Insights
6. Spinal Implants & Surgery Devices Market, by Product Type
7. Spinal Implants & Surgery Devices Market, by Type of Surgery
8. Spinal Implants & Surgery Devices Market, by End-User
9. Americas Spinal Implants & Surgery Devices Market
10. Asia-Pacific Spinal Implants & Surgery Devices Market
11. Europe, Middle East & Africa Spinal Implants & Surgery Devices Market
12. Competitive Landscape
13. Competitive Portfolio
14. Appendix
The report provides insights on the following pointers:
1. Market Penetration: Provides comprehensive information on the market offered by the key players
2. Market Development: Provides in-depth information about lucrative emerging markets and analyzes penetration across mature segments of the markets
3. Market Diversification: Provides detailed information about new product launches, untapped geographies, recent developments, and investments
4. Competitive Assessment & Intelligence: Provides an exhaustive assessment of market shares, strategies, products, certification, regulatory approvals, patent landscape, and manufacturing capabilities of the leading players
5. Product Development & Innovation: Provides intelligent insights on future technologies, R&D activities, and breakthrough product developments
The report answers questions such as:
1. What is the market size and forecast of the Spinal Implants & Surgery Devices Market?
2. Which are the products/segments/applications/areas to invest in over the forecast period in the Spinal Implants & Surgery Devices Market?
3. What is the competitive strategic window for opportunities in the Spinal Implants & Surgery Devices Market?
4. What are the technology trends and regulatory frameworks in the Spinal Implants & Surgery Devices Market?
5. What is the market share of the leading vendors in the Spinal Implants & Surgery Devices Market?
6. What modes and strategic moves are considered suitable for entering the Spinal Implants & Surgery Devices Market?
Read More @ https://www.360iresearch.com/library/intelligence/spinal-implants-surgery-devices?utm_source=einpresswire&utm_medium=referral&utm_campaign=analyst
Mr. Ketan Rohom
360iResearch
+ 1 530-264-8485
ketan@360iresearch.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.