Biopsy Devices Market worth $4.87 billion by 2030, growing at a CAGR of 8.51% - Exclusive Report by 360iResearch
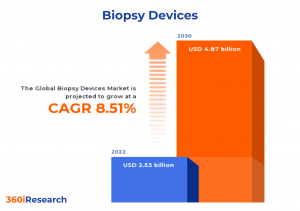
The Global Biopsy Devices Market to grow from USD 2.53 billion in 2022 to USD 4.87 billion by 2030, at a CAGR of 8.51%.
PUNE, MAHARASHTRA, INDIA, November 16, 2023 /EINPresswire.com/ -- The "Biopsy Devices Market by Product (Localization Wires, Needle-Based Biopsy Instruments, Procedure Trays), Technique (MRI-Guided Biopsy, Stereotactic-Guided Biopsy, Ultrasound-Guided Biopsy), Application, End User - Global Forecast 2023-2030" report has been added to 360iResearch.com's offering.The Global Biopsy Devices Market to grow from USD 2.53 billion in 2022 to USD 4.87 billion by 2030, at a CAGR of 8.51%.
Request a Free Sample Report @ https://www.360iresearch.com/library/intelligence/biopsy-devices?utm_source=einpresswire&utm_medium=referral&utm_campaign=sample
The biopsy devices are used explicitly in diagnostic procedures that involve the extraction of sample cells and tissues for examination aimed at identifying the existence and extent of a disease. These devices range from needle biopsy devices, biopsy guidance systems to biopsy forceps. Biopsy devices have a crucial role in several medical applications pertaining to disease detection and monitoring, including breast biopsy, gastrointestinal biopsy, prostate biopsy, liver biopsy, and lung biopsy. An increasing prevalence of cancer worldwide and a growing preference for minimally invasive surgeries are pivotal factors driving the market growth. In contrast, high equipment costs, risks associated with biopsy procedures, and the stringent regulatory hurdles by health authorities hinder the market growth. However, several vendors are focusing on R&D to overcome these issues. Furthermore, advancements in the manufacturing process of biopsy devices, extending the usage of biopsy devices in research and tissue banking, improved healthcare infrastructures, and investments in emerging economies contribute to market expansion.
Technique: Significant role of MRI-guided biopsy in biopsy device for high diagnosis accuracy
MRI-guided biopsy becomes a preferred technique for diagnosing abnormalities located in difficult-to-reach areas or those of more intricate structures such as the brain and the breast. Stereotactic-guided biopsies are more applicable for relatively superficial abnormalities, including those in the breast and lesions close to the skin. This technique is a boon for patients to avoid the discomfort of invasive procedures while still ensuring a highly accurate diagnosis. Ultrasound-guided biopsy primarily leverages its advantage in real-time image provision, making it the go-to option for neck, chest, and abdomen biopsies. Furthermore, it's a cost-effective and faster way of performing biopsies and is preferred in situations where cost and time are primary considerations. In comparison, MRI-guided biopsy stands at the forefront of advanced cases, stereotactic biopsy rings are necessary for superficial cases, and ultrasound-guided offerings shine in real-time requirements and cost-effective scenarios.
Application: Growing need for breast and prostate biopsy devices due to increasing instances of cancer in both areas
The upsurge in breast cancer cases worldwide has subsequently triggered need for breast biopsy devices. Devices such as vacuum-assisted biopsy (VAB) systems attain favor for their minimally invasive nature, confident diagnosis, and high-speed, painless process. Flexible sigmoidoscopes are preferred for colorectal biopsy due to their ability to detect abnormalities in the rectum and sigmoid colon. The rising incidence of lung conditions, including cancer, triggers the need for lung biopsy devices, where transthoracic needle biopsy (TTNB) devices are commonly used. Prostate biopsy procedures largely use transrectal ultrasound (TRUS) guided prostate biopsy devices, as per need-based preference. Biopsy devices for breast and prostate biopsies are undergoing significant technological advancement owing to rising cancer rates. Simultaneously, devices for lung and colorectal biopsies address a range of illnesses, cancer included. Biopsy devices remain paramount in medical diagnosis, reflecting continuous progress and ensuring patient health.
Product: Consumer preference for needle-based biopsy instruments as it provide minimal risk of complications
Localization wires are essential to guide the removal of non-palpable breast lesions during a surgical biopsy. They enable surgeons to locate and accurately extract abnormal tissue effortlessly. Needle-based biopsy instruments are crucial in procuring tissue samples for determining the presence and extent of a disease. They are favored for their less invasive nature, quick recovery time, and minimal risk of complications. Core biopsy devices are crucial biopsy instruments frequently used to obtain tissue samples for pathological examination, primarily to diagnose or rule out cancer. Core biopsy guns are needle-based biopsy instruments that employ a loaded spring mechanism to rapidly advance the biopsy needle into tissue for core sampling, with the significant advantage of minimal tissue displacement. Core biopsy needles assist in extracting core tissue samples that are larger than those acquired through fine needle biopsy. Vacuum-assisted biopsy devices are modern needle-based methods used to extract tissue samples. Vacuum-assisted biopsy needles are a relatively new development in biopsy technology. These needles enable larger and multiple tissue samples to be captured from a single insertion point. Vacuum-assisted biopsy systems present an entirely integrated solution that includes vacuum-assisted biopsy needles. They often incorporate imaging guidance to target abnormal cells and tissues precisely. The systems provide real-time image-guided removal of tissue samples through the vacuum mechanism, allowing for a faster, single-insertion biopsy, improving patient comfort and reducing procedure time. Procedure trays are vital in maintaining surgical efficiency, reducing preparation time, and ensuring patient safety by minimizing infection risk. They contain all the necessary tools and consumables required for varying forms of biopsies. In comparison localization wires emphasize patient comfort and accurate lesion targeting, needle-based biopsy instruments are selected based on the needle size and the lesion type, while procedure trays are chosen depending on the surgical workflow and sterilization methods.
End User: Rising utilization of biopsy devices that promote lesser recovery time in the hospitals
Academic and research institutes pursue innovative solutions for improving the accuracy and efficiency of biopsy procedures, with a particular emphasis on non-invasive techniques. These institutions seek sophisticated and advanced biopsy devices that allow them to explore new frontiers of medical research. Diagnostic and imaging centers focus primarily on timely and accurate diagnosis. They require biopsy devices that facilitate rapid sample collection with minimal patient discomfort. As primary healthcare providers, hospitals focus on patient safety, comfort, and cost-effectiveness. Biopsy devices that allow for shorter recovery times, fewer complications, and lower costs are ideally preferred. The shared objective across these user groups is to enhance biopsy procedure accuracy and efficiency. The varied sector needs to drive market evolution, promising more nuanced innovations and improved global health outcomes.
Regional Insights:
The American region has witnessed a rising trend in the utilization of biopsy devices due to the reduction of various cancer cases. According to the American Cancer Society, in 2023, there are estimated to be 1,958,310 new cancer cases. This directly contributes to the need for biopsy devices. State-of-the-art technology and medical advancements, alongside favorable reimbursement policies in this region, contribute to the market growth. Also, growing awareness about early detection of cancer, reinforced by strong healthcare marketing, paves the path for increased use of biopsy devices in the region. Furthermore, the EMEA region, characterized by rapidly aging populations and a high incidence of cancer, has a strong need for biopsy devices. The market inflow is also credited to the adopting of technologically advanced healthcare equipment and infrastructure. National healthcare systems in these regions promote timely and accurate cancer diagnosis, enabling increased biopsy device utilization. The awareness campaigns by the World Health Organization (WHO) and the European Institute of Oncology stimulate the need for these devices in the region. Moreover, the APAC region is experiencing a rapid increase in lifestyle-related diseases, including cancers. The aging population, environmental pollution, and unhealthy lifestyle habits are fueling the incidences of cancer, thereby increasing the need for biopsy devices. In addition, improving healthcare systems, increasing healthcare spending, and raising awareness about early cancer detection are other factors fostering the adoption of biopsy devices.
FPNV Positioning Matrix:
The FPNV Positioning Matrix is essential for assessing the Biopsy Devices Market. It provides a comprehensive evaluation of vendors by examining key metrics within Business Strategy and Product Satisfaction, allowing users to make informed decisions based on their specific needs. This advanced analysis then organizes these vendors into four distinct quadrants, which represent varying levels of success: Forefront (F), Pathfinder (P), Niche (N), or Vital(V).
Market Share Analysis:
The Market Share Analysis offers an insightful look at the current state of vendors in the Biopsy Devices Market. By comparing vendor contributions to overall revenue, customer base, and other key metrics, we can give companies a greater understanding of their performance and what they are up against when competing for market share. The analysis also sheds light on just how competitive any given sector is about accumulation, fragmentation dominance, and amalgamation traits over the base year period studied.
Key Company Profiles:
The report delves into recent significant developments in the Biopsy Devices Market, highlighting leading vendors and their innovative profiles. These include Angiotech Pharmaceuticals, Inc., Argon Medical Devices, Inc., B. Braun Melsungen AG, Becton, Dickinson and Company, Boston Scientific Corporation, Cardinal Health, Inc., CONMED Corporation, Cook Group Incorporated, Danaher Corporation, DTR Medical Ltd. by Innovia Medical, Fujifilm Holdings Corporation, Hologic, Inc., INRAD, Inc., Intact Medical Corporation, Integra LifeSciences Corporation, Leica Biosystems Nussloch GmbH, Medtronic PLC, Merit Medical Systems, Olympus Corporation, Stryker Corporation, TransMed7, LLC, and TSK Laboratory Europe BV.
Inquire Before Buying @ https://www.360iresearch.com/library/intelligence/biopsy-devices?utm_source=einpresswire&utm_medium=referral&utm_campaign=inquire
Market Segmentation & Coverage:
This research report categorizes the Biopsy Devices Market in order to forecast the revenues and analyze trends in each of following sub-markets:
Based on Product, market is studied across Localization Wires, Needle-Based Biopsy Instruments, and Procedure Trays. The Needle-Based Biopsy Instruments is further studied across Core Biopsy Devices and Vacuum-Assisted Biopsy Devices. The Core Biopsy Devices is further studied across Core Biopsy Guns and Core Biopsy Needles. The Vacuum-Assisted Biopsy Devices is further studied across Vacuum-Assisted Biopsy Needles and Vacuum-Assisted Biopsy Systems. The Procedure Trays is projected to witness significant market share during forecast period.
Based on Technique, market is studied across MRI-Guided Biopsy, Stereotactic-Guided Biopsy, and Ultrasound-Guided Biopsy. The Ultrasound-Guided Biopsy is projected to witness significant market share during forecast period.
Based on Application, market is studied across Breast Biopsy, Colorectal Biopsy, Lung Biopsy, and Prostate Biopsy. The Lung Biopsy is projected to witness significant market share during forecast period.
Based on End User, market is studied across Academic & Research Institutes, Diagnostic & Imaging Centers, and Hospitals. The Hospitals is projected to witness significant market share during forecast period.
Based on Region, market is studied across Americas, Asia-Pacific, and Europe, Middle East & Africa. The Americas is further studied across Argentina, Brazil, Canada, Mexico, and United States. The United States is further studied across California, Florida, Illinois, New York, Ohio, Pennsylvania, and Texas. The Asia-Pacific is further studied across Australia, China, India, Indonesia, Japan, Malaysia, Philippines, Singapore, South Korea, Taiwan, Thailand, and Vietnam. The Europe, Middle East & Africa is further studied across Denmark, Egypt, Finland, France, Germany, Israel, Italy, Netherlands, Nigeria, Norway, Poland, Qatar, Russia, Saudi Arabia, South Africa, Spain, Sweden, Switzerland, Turkey, United Arab Emirates, and United Kingdom. The Americas commanded largest market share of 38.74% in 2022, followed by Europe, Middle East & Africa.
Key Topics Covered:
1. Preface
2. Research Methodology
3. Executive Summary
4. Market Overview
5. Market Insights
6. Biopsy Devices Market, by Product
7. Biopsy Devices Market, by Technique
8. Biopsy Devices Market, by Application
9. Biopsy Devices Market, by End User
10. Americas Biopsy Devices Market
11. Asia-Pacific Biopsy Devices Market
12. Europe, Middle East & Africa Biopsy Devices Market
13. Competitive Landscape
14. Competitive Portfolio
15. Appendix
The report provides insights on the following pointers:
1. Market Penetration: Provides comprehensive information on the market offered by the key players
2. Market Development: Provides in-depth information about lucrative emerging markets and analyzes penetration across mature segments of the markets
3. Market Diversification: Provides detailed information about new product launches, untapped geographies, recent developments, and investments
4. Competitive Assessment & Intelligence: Provides an exhaustive assessment of market shares, strategies, products, certification, regulatory approvals, patent landscape, and manufacturing capabilities of the leading players
5. Product Development & Innovation: Provides intelligent insights on future technologies, R&D activities, and breakthrough product developments
The report answers questions such as:
1. What is the market size and forecast of the Biopsy Devices Market?
2. Which are the products/segments/applications/areas to invest in over the forecast period in the Biopsy Devices Market?
3. What is the competitive strategic window for opportunities in the Biopsy Devices Market?
4. What are the technology trends and regulatory frameworks in the Biopsy Devices Market?
5. What is the market share of the leading vendors in the Biopsy Devices Market?
6. What modes and strategic moves are considered suitable for entering the Biopsy Devices Market?
Read More @ https://www.360iresearch.com/library/intelligence/biopsy-devices?utm_source=einpresswire&utm_medium=referral&utm_campaign=analyst
Mr. Ketan Rohom
360iResearch
+ 1 530-264-8485
ketan@360iresearch.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.