Biosimulation Market worth $9.87 billion by 2030, growing at a CAGR of 14.71% - Exclusive Report by 360iResearch
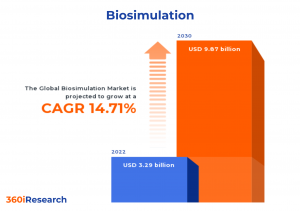
The Global Biosimulation Market to grow from USD 3.29 billion in 2022 to USD 9.87 billion by 2030, at a CAGR of 14.71%.
PUNE, MAHARASHTRA, INDIA, November 10, 2023 /EINPresswire.com/ -- The "Biosimulation Market by Offering (Services, Software), Delivery Model (Ownership Models, Subscription Models), End-User, Application - Global Forecast 2023-2030" report has been added to 360iResearch.com's offering.The Global Biosimulation Market to grow from USD 3.29 billion in 2022 to USD 9.87 billion by 2030, at a CAGR of 14.71%.
Request a Free Sample Report @ https://www.360iresearch.com/library/intelligence/biosimulation?utm_source=einpresswire&utm_medium=referral&utm_campaign=sample
Biosimulation combines mathematics, biology, and computer programming to create simulations to test hypotheses, predict outcomes, and explore new possibilities in biotechnology. By simulating biological phenomena such as gene expression, metabolism, and cell growth, biosimulation helps researchers better understand the complexities of life and develop new products for healthcare. The increasing development of new drugs and medicines and the growing government focus on integrating digitalized technologies in pharmaceutical and biopharmaceutical sectors are elevating the need for biosimulation systems. However, the high cost associated with developing biosimulation software hampers market growth. The growing integration of computer-aided design (CAD) and virtual reality (VR) technologies, as well as advancements in artificial intelligence (AI) and machine learning (ML) for the development of new biosimulation software, is expected to expand the scope of the biosimulation market.
Offering: Rising usage of software in biosimulation to provide a platform for model building, simulation, analysis, and visualization
Contract services are provided by specialized companies and research organizations that offer biosimulation expertise to clients, which assist with model development, simulation studies, data analysis, and interpretation. In-house services are biosimulation services conducted within an organization and research institution. Molecular modeling & simulation software focuses on simulating and modeling the behavior of molecules, such as proteins, nucleic acids, and small molecules. It is used to study molecular interactions and conformational changes and predict properties such as binding affinity. PBPK modeling & simulation software is designed to simulate the distribution, absorption, metabolism, and excretion of drugs and chemicals in the body. PK/PD, modeling & simulation software, affects drugs' pharmacokinetic and pharmacodynamic properties, helps predict drug concentrations and exposure-response relationships, and optimizes dosing regimens. Toxicity prediction software focuses on predicting the potential toxicity of chemical compounds. It uses computational models to assess the safety and potential risks of exposure to specific chemicals or drugs. Trial design software helps in the design and optimization of clinical trials and also simulates different trial scenarios, evaluates statistical power, and optimizes sample sizes to improve the efficiency and validity of clinical studies.
Application: Increasing application of biosimulation for drug development
Biosimulation plays a vital role in drug development by providing insights and predictions related to drug pharmacokinetic (PK) and pharmacodynamic (PD) properties, assisting in optimizing dosing regimens, predicting drug concentrations in target tissues, and assessing drug candidates' potential efficacy and safety. Biosimulation techniques, such as PBPK and PK/PD modeling, aid in making informed decisions during drug development. Biosimulation supports the design and optimization of clinical trials and enables the prediction and evaluation of drugs and chemical compounds' absorption, distribution, metabolism, excretion, and toxicity properties. Biosimulation helps identify potential ADME/Tox issues early in the drug development process, reducing the need for extensive animal testing and providing insights into potential risks. PK/PD modeling and simulation assist in predicting the relationship between drug exposure and response. Biosimulation supports lead identification & optimization by employing molecular modeling and simulation techniques. It aids in screening and evaluating potential drug candidates, predicting their binding affinity to target molecules, and optimizing their chemical structures for improved potency and selectivity. Biosimulation techniques aid in the identification and validation of potential drug targets. By modeling and simulating the interactions between drug molecules and target proteins, biosimulation helps evaluate the feasibility and potential effectiveness of targeting specific proteins.
Delivery Model: Growing preference for ownership model in biosimulation for providing full control to users
In the biosimulation ownership model, users purchase the software and service outright, giving them full control over the software and its usage. In the biosimulation ownership model, users purchase a perpetual license for the software, granting them the right to use it indefinitely. Subscription models involve users paying a recurring fee to access and use the software or services. With this model, users usually do not own the software but have ongoing access to the latest versions and updates.
End-User: Expanding adoption of biosimulation among pharmaceutical & biotechnology companies
Contract research organizations (CROs) often utilize biosimulation to support their clients' drug discovery and development processes and offer services such as molecular modeling, PK/PD modeling, toxicity prediction, and trial design using biosimulation techniques. Pharmaceutical & biotechnology companies extensively employ biosimulation throughout the drug development pipeline. Regulatory authorities, including the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), play a crucial role in evaluating drug safety and effectiveness before being approved for market release. Regulatory authorities use biosimulation to assess the risk-benefit profile of drugs, optimize study designs, and make informed decisions during the drug approval process. Research institutes, including academic institutions and government organizations, employ biosimulation to advance scientific knowledge and conduct advanced research. Researchers utilize biosimulation tools and techniques to model and simulate complex biological systems, explore hypotheses, and gain insights into fundamental biological processes.
Regional Insights:
The Americas region showcases a highly developing landscape for the biosimulation market owing to the presence of major established players in the United States and their ongoing efforts to advance biosimulation software. Well-established regulatory authorities such as the Food and Drug Administration (FDA), European Medicines Agency (EMA), and Health Canada to ensure the safety and efficiency of drug development and clinical trials are benefiting the market growth across the Americas, Europe, and Asia-Pacific. Considering the EMEA region, the increasing investment in drug development and clinical trials across UK, Germany, and France is expanding the adoption of biosimulation software & services. The increasing number of chronic diseases across Asia-Pacific encourages government and regulatory authorities to expand their funding activities for drug development which is expected to create a platform for developing the biosimulation market. The presence of various initiatives such as Australia's Drug Discovery Initiative and Medical Research Commercialisation Initiative that aims at pharmaceutical research and development (R&D) activities is expanding the usage of biosimulation in Asia-pacific.
FPNV Positioning Matrix:
The FPNV Positioning Matrix is essential for assessing the Biosimulation Market. It provides a comprehensive evaluation of vendors by examining key metrics within Business Strategy and Product Satisfaction, allowing users to make informed decisions based on their specific needs. This advanced analysis then organizes these vendors into four distinct quadrants, which represent varying levels of success: Forefront (F), Pathfinder (P), Niche (N), or Vital(V).
Market Share Analysis:
The Market Share Analysis offers an insightful look at the current state of vendors in the Biosimulation Market. By comparing vendor contributions to overall revenue, customer base, and other key metrics, we can give companies a greater understanding of their performance and what they are up against when competing for market share. The analysis also sheds light on just how competitive any given sector is about accumulation, fragmentation dominance, and amalgamation traits over the base year period studied.
Key Company Profiles:
The report delves into recent significant developments in the Biosimulation Market, highlighting leading vendors and their innovative profiles. These include Advanced Chemistry Development, Inc., Aitia, Allucent, Applied BioMath, LLC, Biomed Simulation, Inc., BioSimulation Consulting Inc., Cadence Design Systems, Inc., Cell Works Group, Inc., Certara, Inc., Chemical Computing Group ULC, Compugen Ltd., Crystal Pharmatech Co., Ltd., Dassault Systèmes SE, Genedata AG, Immunetrics Inc, In Silico Biosciences, Inc., INOSIM Software GmbH, Instem PLC, Laboratory Corporation of America Holdings, Model Vitals, Physiomics PLC, Quotient Sciences Limited, Resolution Medical, Rosa & Co LLC, Schrodinger, Inc., Simulations Plus, Inc., Thermo Fisher Scientific Inc., VeriSIM Life., VIRTUALMAN, and Yokogawa Electric Corporation.
Inquire Before Buying @ https://www.360iresearch.com/library/intelligence/biosimulation?utm_source=einpresswire&utm_medium=referral&utm_campaign=inquire
Market Segmentation & Coverage:
This research report categorizes the Biosimulation Market in order to forecast the revenues and analyze trends in each of following sub-markets:
Based on Offering, market is studied across Services and Software. The Services is further studied across Contract Services and In-House Services. The Software is further studied across Molecular Modeling & Simulation Software, PBPK Modeling & Simulation Software, PK/PD Modeling & Simulation Software, Toxicity Prediction Software, and Trial Design Software. The Software commanded largest market share of 77.65% in 2022, followed by Services.
Based on Delivery Model, market is studied across Ownership Models and Subscription Models. The Ownership Models commanded largest market share of 60.46% in 2022, followed by Subscription Models.
Based on End-User, market is studied across Contract Research Organizations, Pharmaceutical & Biotechnology Companies, Regulatory Authorities, and Research Institutes. The Pharmaceutical & Biotechnology Companies commanded largest market share of 47.54% in 2022, followed by Contract Research Organizations.
Based on Application, market is studied across Drug Development and Drug Discovery. The Drug Development is further studied across Clinical Trials and Preclinical Testing. The Preclinical Testing is further studied across ADME/Tox and PK/PD. The Drug Discovery is further studied across Lead Identification & Optimization and Target Identification & Validation. The Drug Development commanded largest market share of 53.33% in 2022, followed by Drug Discovery.
Based on Region, market is studied across Americas, Asia-Pacific, and Europe, Middle East & Africa. The Americas is further studied across Argentina, Brazil, Canada, Mexico, and United States. The United States is further studied across California, Florida, Illinois, New York, Ohio, Pennsylvania, and Texas. The Asia-Pacific is further studied across Australia, China, India, Indonesia, Japan, Malaysia, Philippines, Singapore, South Korea, Taiwan, Thailand, and Vietnam. The Europe, Middle East & Africa is further studied across Denmark, Egypt, Finland, France, Germany, Israel, Italy, Netherlands, Nigeria, Norway, Poland, Qatar, Russia, Saudi Arabia, South Africa, Spain, Sweden, Switzerland, Turkey, United Arab Emirates, and United Kingdom. The Americas commanded largest market share of 42.52% in 2022, followed by Europe, Middle East & Africa.
Key Topics Covered:
1. Preface
2. Research Methodology
3. Executive Summary
4. Market Overview
5. Market Insights
6. Biosimulation Market, by Offering
7. Biosimulation Market, by Delivery Model
8. Biosimulation Market, by End-User
9. Biosimulation Market, by Application
10. Americas Biosimulation Market
11. Asia-Pacific Biosimulation Market
12. Europe, Middle East & Africa Biosimulation Market
13. Competitive Landscape
14. Competitive Portfolio
15. Appendix
The report provides insights on the following pointers:
1. Market Penetration: Provides comprehensive information on the market offered by the key players
2. Market Development: Provides in-depth information about lucrative emerging markets and analyzes penetration across mature segments of the markets
3. Market Diversification: Provides detailed information about new product launches, untapped geographies, recent developments, and investments
4. Competitive Assessment & Intelligence: Provides an exhaustive assessment of market shares, strategies, products, certification, regulatory approvals, patent landscape, and manufacturing capabilities of the leading players
5. Product Development & Innovation: Provides intelligent insights on future technologies, R&D activities, and breakthrough product developments
The report answers questions such as:
1. What is the market size and forecast of the Biosimulation Market?
2. Which are the products/segments/applications/areas to invest in over the forecast period in the Biosimulation Market?
3. What is the competitive strategic window for opportunities in the Biosimulation Market?
4. What are the technology trends and regulatory frameworks in the Biosimulation Market?
5. What is the market share of the leading vendors in the Biosimulation Market?
6. What modes and strategic moves are considered suitable for entering the Biosimulation Market?
Read More @ https://www.360iresearch.com/library/intelligence/biosimulation?utm_source=einpresswire&utm_medium=referral&utm_campaign=analyst
Mr. Ketan Rohom
360iResearch
+ +1 530-264-8485
ketan@360iresearch.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.