EmergingGrowth.com Reports on Why VBI Vaccines (NASDAQ: VBIV) Could Be a Contrarian Investor’s Dream
With few appreciating the science behind VBIV stock, this could be an undervalued winner
VBI Vaccines (NASDAQ:VBIV)
MIAMI, FLORIDA, USA, September 30, 2020 /EINPresswire.com/ -- No matter how much social distancing government institutions request (or impose, in many cases), it’s becoming clear that we need a vaccine for a holistic solution to the novel coronavirus pandemic. As you know, so much of our customs depend on high-contact practices, such as shaking hands. Unless we intend to bow to each other like in Asian countries, widespread vaccination is perhaps our most viable answer.Not surprisingly, one of the hottest sectors in the markets this year has been biotechnology. With corporations both big and small pivoting sharply to the research and development of a Covid-19 vaccine, investors have enthusiastically jumped on this bandwagon. After a substantial bout of volatility likely due to profit-taking, sentiment for the vaccine players has returned.
While the list of those garnering the most attention read like the usual suspects, there’s probably one biotech firm you haven’t heard of, VBI Vaccines (NASDAQ:VBIV). While VBIV stock soared to multi-year highs around mid-July, shares have found themselves plummeting back to earth. As several now well-known vaccine companies have entered into late-stage clinical trials for their Covid-19 candidates, the other, smaller names have lost their luster.
However, I think it’s a mistake to ignore VBIV stock. While the underlying company probably won’t be the first one to cross the clinical finish line, this race may not turn out to be a traditional one; that is, this competition could have more than one winner.
Since it’s unlikely that a Covid-19 vaccine will be available until the end of this year at the earliest, the time element isn’t as strong as it used to be. Further, 62% of Americans “are worried that ‘political pressure’ from the Trump administration will rush a coronavirus vaccine.” Safety, as it turns out, is a priority.
Americans are rational actors after all. Moreover, the deeper you drill into the narrative for the other vaccines, the more you realize that this race is still very much open.
Vaccines Feature a Startling Mixture of Pros and Cons
While the types of vaccines proposed to combat the novel coronavirus are diverse, the major formats are as follows: traditional, subunit, viral vector and nucleic acids.
First, the traditional type is the one with which we are most familiar. This process involves injecting a weakened or inactivated (dead) form of the target virus into the patient. From there, the patient’s cell takes the genetic information from the weakened/inactivated virus and initiates a process which creates antibodies. Antibodies are large Y-shaped proteins that attach to pathogens, preventing them from infecting healthy cells, thus neutralizing them.
Obviously, traditional vaccines have the longest track record of success. So, if we were judging a Covid-19 vaccine purely on efficacy, the traditional format would win out. Unfortunately, these vaccines take significant time to manufacture, which effectively rules them out.
Second, we have subunit vaccines. This process involves injecting fragments (subunits) of the novel coronavirus proteins into the patient. From there, the patient’s cells take the information from the spike proteins and initiates the process to develop antibodies.
Admittedly, subunits are compelling because they are proven to work. For instance, Recombivax HB is a Hepatitis B subunit vaccine. Further, subunits are especially appropriate for patients with underlying conditions due to their safety profile. Clearly, those who have the worst outcomes from Covid-19 are immune-compromised patients. Thus, companies like Novavax (NASDAQ:NVAX) have adopted subunits as their go-to platform.
Unfortunately, the manufacturing process to develop the nanoparticles to make these vaccines are much more complicated and expensive. Further, the complexity may require Novavax to restructure or build out more physical capacity. That could be a problem if a second wave emerges, necessitating urgency.
Third, viral-vector vaccines offer solutions that almost come out of a science fiction movie. Here, the novel coronavirus gene is placed inside a carrier (or vector, hence the name) virus (typically an adenovirus). This is then injected into the patient. The carrier virus infects a cell, but rather than killing it, the vector provides the cell with the genetic sequence of the target virus. As with the other vaccine types, the cell initiates antibody production.
Viral vectors form some of the corner blocks of gene therapy. By using the infectious nature of the adenovirus for positive health outcomes, scientists hope to address multiple genetic conditions. But the main problem with viral vectors is that patients can develop an immunoresponse against the carrier virus.
That may have been – although it’s unconfirmed – the hiccup that AstraZeneca (NYSE:AZN) incurred with its Covid-19 vaccine candidate, when a U.K. patient came down with an “unexplained illness.” While trials have resumed, many people are understandably concerned about viral vectors now.
Finally, we have nucleic-acid based vaccines. Currently, Moderna (NASDAQ:MRNA) leads this space with its messenger RNA (mRNA) solution. Here, bioengineers manufacture mRNA in a lab, placing it inside a lipid shell. This is injected into a patient’s cell, where the mRNA provides the “recipe” for coronavirus antibody production.
Compared to other vaccine types, nucleic-acid vaccines can be easily manufactured, presenting a solution for the scale problem. However, this format is the most experimental, with no nucleic-acid vaccines having been approved by the Food and Drug Administration. Therefore, if Moderna succeeds, it will be history in the making.
Why VBI Vaccines Shines Bright
Based on all scientific and administrative factors, it appears that Moderna is in the lead. However, its mRNA vaccine does have one more issue that I haven’t mentioned yet: titers or lack thereof.
According to a preliminary report from the New England Journal of Medicine regarding Moderna’s vaccine candidate, antibody titers increased dramatically between the first and second vaccinations in trial participants. This implies that mRNA-based vaccines will require two dosages. Unfortunately, this will be costly, inefficient and inconvenient to the patient.
What we really need is a one-and-done solution. And that’s where VBI Vaccines comes into the picture with its enveloped virus-like particle (eVLP) approach. Similar to a traditional vaccine, an eVLP provides the genetic sequence of the target virus to the cell. But the key difference here is that eVLP involves only the spike protein, in this case the “sticky” portion of the novel coronavirus.
Most importantly, what VBI’s eVLP approach offers is high antibody titers. That’s because the high surface-to-volume ratio of the small particles associated with eVLP vaccines enables the delivery of several copies of the spike protein. Essentially, one eVLP can bind to multiple cells, initiating a wave of antibody production.
Further, research from the Wuhan Institute of Virology notes that eVLP vaccines feature self-adjuvanting properties, an advantage over subunit vaccines. Best of all, eVLPs are known for their safety and simplicity, attributes that could make it a big winner in the Covid vaccine race.
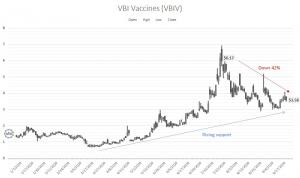
From this year’s closing high, VBIV stock is at time of writing down 42%. Frankly, the market may be irrationally driving shares down. If you consider the science of the underlying company, you’ll recognize that this one-dose vaccination approach is incredibly relevant. Further, even if it doesn’t win the Covid battle, the PR generated for eVLPs could position VBI for upside in 2021 and beyond.
True, I would be remiss not to point out that eVLPs have bioprocessing challenges and also face regulatory scrutiny for their experimental nature. Basically, the disadvantages are similar to nucleic-acid vaccines.
However, the best nucleic-acid candidates will likely require two doses. In sharp contrast, eVLPs will only require one dose (assuming successful manufacturing). That’s the only reason it’s in the conversation right now but it’s a powerful one. With VBIV flying under the radar, now may be a good time to consider a speculative long position.
As of this writing, Josh Enomoto did not hold a position in the aforementioned securities. He is considering a position in VBIV in the next 72 hours.
About EmergingGrowth.com
EmergingGrowth.com is a leading independent small cap media portal with an extensive history of providing unparalleled content for the Emerging Growth markets and companies. Through its evolution, EmergingGrowth.com found a niche in identifying companies that can be overlooked by the markets due to, among other reasons, trading price or market capitalization. We look for strong management, innovation, strategy, execution, and the overall potential for long- term growth. Aside from being a trusted resource for the Emerging Growth info-seekers, we are well known for discovering undervalued companies and bringing them to the attention of the investment community. Through our parent Company, we also have the ability to facilitate road shows to present your products and services to the most influential investment banks in the space.
This article was written by a guest contributor and solely reflects his/her opinions. All information contained herein as well as on the EmergingGrowth.com website is obtained from sources believed to be reliable but not guaranteed to be accurate or all-inclusive. The statements in this article are not that of, nor have they been verified by, or are the opinion of, EmergingGrowth.com. All material is for informational purposes only, and should not be construed as an offer or solicitation to buy or sell securities. The information includes certain forward-looking statements, which may be affected by unforeseen circumstances and / or certain risks. Please consult an investment professional before investing in anything viewed within.
Emerging Growth Staff
EmergingGrowth.com
305-330-1985
email us here
Visit us on social media:
Facebook
Twitter
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.