Genetic Technologies Limited (ASX Code: GTG; NASDAQ Ticker: GENE) announces successful completion of two new ground-breaking Genetic Risk Tests – for Colorectal Cancer and for Breast Cancer
MELBOURNE, Australia, May 10, 2019 (GLOBE NEWSWIRE) -- Genetic Technologies Limited (ASX: GTG; NASDAQ Ticker: GENE) is delighted to announce that following substantial scientific research and genetic product development, two new ground-breaking cancer risk assessment tests have now been completed and validated. The tests are branded as ‘GeneType for Colorectal Cancer’ and ‘GeneType for Breast Cancer’ – see below:
|
||||||||||||||||||||
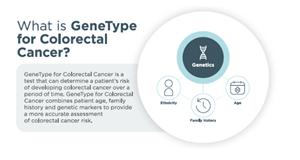
Image 1, What is GeneType for Colorectal Cancer?: http://www.globenewswire.com/NewsRoom/AttachmentNg/a20ba1cf-ead8-4047-bb31-be67a8a91aa3
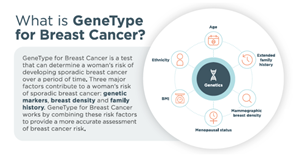
Image 2, What is GeneType for Breast Cancer?: http://www.globenewswire.com/NewsRoom/AttachmentNg/cefef8c1-5543-430a-ab3c-c0a7144600fc
The new tests for colorectal cancer and breast cancer are world-leading.
GTG’s polygenic risk tests combine information from genetic markers called Single Nucleotide Polymorphisms to assess how people’s genetic make-up affects their risk of developing various diseases. GTG’s heavily patented approach to incorporating further clinical risk information places them at the forefront of the global risk assessment space.
The new breast cancer test provides substantial improvement over GTG’s legacy breast cancer test (which was called BREVAGenplus) by incorporating multiple additional clinical risk factors. This test will provide healthcare providers and their patients with a 5-year and lifetime risk assessment of the patient developing breast cancer.
The world-first polygenic risk test for colorectal cancer provides healthcare providers and their patients a 5-year, 10-year, and lifetime risk assessment of the patient developing colorectal cancer.
Importantly, GTG’s new risk tests and the resulting detailed healthcare provider and patient reports empower those people at increased risk to make specific lifestyle changes to reduce their particular risks and improve ongoing early future disease detection.
GTG Chairman and Acting CEO Dr Paul Kasian said, “These tests allow Government Health Leaders to directly target future screening to the most high-risk patients, thereby massively reducing health system costs and providing much better outcomes for patients. GTG’s proactive approach to disease management has the potential to save both lives and money by allowing the earlier detection of disease and focusing the use of limited healthcare resources to those most in need.”
Dr Kasian added, “I am very proud of our world-class scientific team. Genetic testing is a growing industry world-wide and it is remarkable that the team has maintained thought and product development leadership in this competitive space. Armed with these new world class products, GTG is poised to play its part in making predictive genetic testing a mainstream healthcare activity – a routine part of any health check-up.”
New Product – ‘GeneType for Colorectal Cancer’
Next generation risk assessments combine multiple clinical and genetic risk factors to better stratify individuals at increased risk of developing disease. ‘GeneType for Colorectal Cancer’ incorporates the most impactful risk factors in order to define an individual’s risk of developing colorectal cancer, so the healthcare provider can make screening and preventative care recommendations that are tailored to their patient’s personalised risk.
Colorectal cancer is the 3rd most commonly diagnosed cancer in the US, yet 1 in 3 adults are not receiving the appropriate colorectal cancer screening for their age. In addition, rates of colorectal cancer among 20-49 year olds is steadily increasing. Identifying patients who are most at risk for colorectal cancer can lead to enhanced screening protocols and better outcomes. Most individuals diagnosed with colorectal cancer do not have a significant family history of the disease. ‘GeneType for Colorectal Cancer’ evaluates the genometric risk of developing colorectal cancer for men and women over age 30 who do not have a known pathogenic gene variant.
Polygenic risk identifies patients at unusually high risk of disease
In sporadic colorectal cancer, no single gene mutation is causal of disease. Rather, common DNA variations, called single nucleotide polymorphisms (SNPs), each contribute a small but measurable risk of developing disease. ‘GeneType for Colorectal Cancer’ analyzes a patient’s DNA for 40+ SNPs that have been clinically validated in their association with colorectal cancer. By combining the effects of all of these SNPs into a single polygenic risk score (PRS), ‘GeneType for Colorectal Cancer’ provides a superior risk stratification over standard risk assessments that incorporate only clinical factors.
‘GeneType for Colorectal Cancer’ is clinically validated for men and women 30 years of age or older and for individuals of Caucasian descent. Genetic Technologies will provide updates as we continuously improve our test and add fully validated models for additional ethnicities.
New Product – ‘GeneType for Breast Cancer’
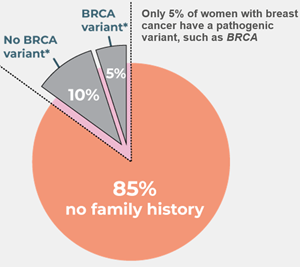
Germline genetic testing for mutations in BRCA1 and BRCA2 allows for the identification of individuals at significantly increased risk for breast and other cancers. However, such mutations are relatively rare in the general population and account for less than 10% of all breast cancer cases. The remaining 90% of non-familial or sporadic breast cancer have to be defined by other genetic/clinical markers common to the population at large and this is where Genetic Technologies has focused its attention.
The newly developed ‘GeneType for Breast Cancer’ test is aimed at risk detection of non-BRCA related sporadic breast cancer (that is, for those women who do not have an identified family history of breast cancer). Importantly, this means that Genetic Technologies’ test covers 95% of women - see below:
Image 3, Detecting non-BRCA related sporadic breast cancer: http://www.globenewswire.com/NewsRoom/AttachmentNg/6bb27727-a78f-4934-8782-964edbb2b4bb
The key features of Genetic Technologies’ new ‘GeneType’ tests are as follows:
- they are based upon world-leading science
- patients identified as being at increased risk can be offered increased ongoing targeted screening, protecting them against the poor health outcomes and high costs from late diagnosis of disease
- GTG’s scientific team has perfected concise patient questionnaires, so as to minimize clinical consultation times for busy health practitioners (lengthy time-consuming questionnaires have been a widespread weakness with non-Genetic Technologies sourced polygenic risk score tests
- unlike many other genetic tests, GTG’s products are modest in price, making them affordable to all so that they can become a key part of regular healthcare provider health checks and a core component of Government health cost reduction programs
- importantly, the tests also empower healthcare providers to work with patients on lifestyle changes which can reduce both colorectal and breast cancer risk
- the Intellectual Property is supported by a strong global (including the US) patent portfolio
Commercialization and Product Distribution Plans
GTG intends to introduce the new ‘GeneType for Colorectal Cancer’’ and ‘GeneType for Breast Cancer’ genetic tests to healthcare providers through a global network of distribution partners. The Company has already announced it is working with TGen in the United States and also Chinese partners connected to the Hainan Free-Trade Zone. More announcements are anticipated in this regard in coming months as GTG moves to commercialize its suite of world-leading genetic tests.
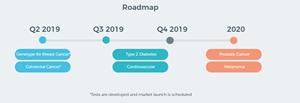
Following the successful completion of the ‘GeneType for Colorectal Cancer’ and ‘GeneType for Breast Cancer’ genetic tests, GTG’s Scientific and Product Development Team will refocus on the future pipeline of exciting new tests detailed below and as previously announced to the market.
Image 4, New Test Pipeline: http://www.globenewswire.com/NewsRoom/AttachmentNg/8aeb5a80-3cea-4066-9fd6-e27e7b6e0e8f
FOR FURTHER INFORMATION PLEASE CONTACT
Dr Paul Kasian
Chairman and CEO
Genetic Technologies
+61 3 8412 7000
Mr Paul Viney
COO, CFO and Company Secretary
Genetic Technologies
+61 438 072 616
Investor Relations and Media (Australia)
Ms Karinza Phoenix
StocksDigital
+61 428 981 074
karinza@stocksdigital
Investor Relations and Media (US)
Mr Dave Gentry
RedChip
Office: 1 800 RED CHIP (733 2447)
Cell: 407 491 4498
dave@redchip.com
About Genetic Technologies Limited
Genetic Technologies Limited (ASX: GTG; Nasdaq: GENE) is a diversified molecular diagnostics company. GTG offers cancer predictive testing and assessment tools to help physicians proactively manage patient health. The Company lead products GeneType for Breast Cancer and ‘GeneType for Colorectal Cancer’ are clinically validated risk assessment tests for non-hereditary breast cancer and are first in class.
Genetic Technologies is developing a pipeline of risk assessment products.
For more information, please visit www.gtglabs.com
Safe Harbor Statement
Any statements in this press release that relate to the Company's expectations are forward-looking statements, within the meaning of the Private Securities Litigation Reform Act. The Private Securities Litigation Reform Act of 1995 (PSLRA) implemented several significant substantive changes affecting certain cases brought under the federal securities laws, including changes related to pleading, discovery, liability, class representation and awards fees. Since this information may involve risks and uncertainties and are subject to change at any time, the Company's actual results may differ materially from expected results. Additional risks associated with Genetic Technologies' business can be found in its periodic filings with the SEC.
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.