Medical Device Validation & verification Market Expected to Hit USD 9.51 Billion by 2034 with a Remarkable 8.18% CAGR
Medical Device Validation & Verification Market is witnessing a transformative growth phase—propelled by regulatory rigor, technological complexity.
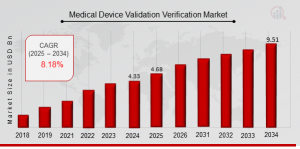
US, NY, UNITED STATES, August 6, 2025 /EINPresswire.com/ -- Medical Device Validation & verification Market is entering a phase of accelerated growth, spurred by intensifying regulatory scrutiny, the proliferation of complex devices, and mounting demand for quality assurance across the healthcare value chain. As per Market Research Future (MRFR), the market size is projected to nearly double—from approximately USD 4.68 billion in 2025 to USD 9.51 billion by 2034, at a robust compound annual growth rate (CAGR) of around 8.18% over the forecast period.Request To Free Sample of This Strategic Report: https://www.marketresearchfuture.com/sample_request/33243
Industry Size and Market Scope
The V&V industry encompasses a comprehensive suite of services—ranging from software validation and hardware verification to clinical testing, documentation audit, compliance reviews and integrated combination V&V offerings. This broad market scope is critical for ensuring medical device safety, efficacy and regulatory adherence ahead of market entry. The industry size, currently anchored in the mid‑single billions, continues expanding as device complexity increases and global regulators demand higher standards of pre‑market and post‑market oversight.
Market Segmentation and Segment Growth
The Medical Device Validation and Verification Market is segmented across multiple dimensions including validation type, service type, device category, end-use, and region. Based on validation type, the market is categorized into design validation, process validation, product verification, and clinical validation—each playing a critical role in ensuring medical device safety, performance, and regulatory compliance throughout the product lifecycle. In terms of service type, the market includes consulting services, testing services, certification services, and audit services, which are essential for guiding manufacturers through the complex regulatory and quality assurance landscape.
Looking at device category, the validation and verification processes are applied to a broad range of medical technologies including in-vitro diagnostics, surgical instruments, implantable devices, and monitoring equipment. These categories represent core areas where strict compliance with validation standards is critical to patient safety and clinical efficacy.
From the perspective of end-use, the market serves a diverse customer base that includes hospitals, clinics, research laboratories, and pharmaceutical companies. These stakeholders depend on reliable validation and verification to ensure device performance and regulatory adherence.
Geographically, the Medical Device Validation and Verification Market is segmented into key regions including North America, Europe, South America, Asia-Pacific, and the Middle East and Africa, with each region presenting distinct regulatory environments and growth opportunities.
You Can Purchase Complete Report: https://www.marketresearchfuture.com/checkout?currency=one_user-USD&report_id=33243
Key Companies in the Medical Device Validation and Verification Market Include
Siemens Healthineers
GE Healthcare
Cardinal Health
Fujifilm
Boston Scientific
Medtronic
Abbott Laboratories
Hitachi
Stryker
Thermo Fisher Scientific
Philips
Baxter International
Arjo
Johnson and Johnson
3M
Source: https://www.marketresearchfuture.com/reports/medical-device-validation-verification-market-33243
Emerging Trends and Opportunities
The adoption of digital V&V platforms, AI‑powered analytics, and automated testing workflows is transforming traditional service delivery. These emerging trends improve efficiency, accuracy, and turnaround time, positioning service providers to capture more business with lower overhead.
Cybersecurity testing, software-as-a‑medical‑device (SaMD) validation, and regulatory consulting are areas witnessing growing demand—creating fresh opportunities for firms that offer integrated, value‑added compliance services.
Growing regulatory divergence across geographies is prompting increased outsourcing by device manufacturers, further expanding the opportunity space for niche V&V specialists.
Recent Developments
Recent market developments cited by MRFR include strategic expansions by leading validation firms: addition of regional laboratory facilities, attainment of new certifications, and deployment of digital compliance tools to track validation workflows and clinical documentation. These recent developments are strengthening the infrastructure and client access to high‑quality V&V services across global markets.
Regional Market Share Insights
North America continues to dominate the market share, attributed to its stringent regulatory framework (FDA), technological sophistication, and concentration of medical device innovation hubs. Meanwhile, Asia-Pacific is expected to deliver the fastest growth, propelled by expanding device manufacturing in China, India, and South Korea, evolving regulatory alignment, and surging demand for cost‑effective V&V solutions. MRFR highlights that Asia‑Pacific’s developing compliance infrastructure makes it a key growth region for stakeholders expanding global service footprints.
Browse In-depth Market Research Report (Pages, Charts, Tables, Figures): https://www.marketresearchfuture.com/reports/medical-device-validation-verification-market-33243
Future Outlook
The future outlook for the Medical Device V&V Market is decidedly positive. With medical devices increasingly integrating software, connectivity, and IoT features, regulators worldwide are tightening validation requirements. The shift toward value‑based healthcare, alongside rising public and provider focus on patient safety, positions comprehensive validation and verification as non‑negotiable. From 2025 to 2034, the market is expected to nearly double, reaching USD 9.51 billion by 2034 as compared to USD 4.68 billion in 2025.
Stakeholder Takeaways
For medical device manufacturers, procurement heads, hospital administrators, distributors, and investors, the V&V market represents a strategic growth arena:
Manufacturers and procurement leaders should examine opportunities to partner with established and emerging V&V service firms to accelerate time‑to‑market and compliance efficiency.
Hospital administrators can benefit from alignment with trusted V&V partners when procuring complex diagnostic and therapeutic devices, ensuring regulatory compliance and patient safety.
Investors and distributors can identify growth-ready service providers offering differentiated digital platforms, cybersecurity validation, and regional coverage—positioning themselves at the forefront of an evolving, compliance-driven market.
Browse More Reports:
Transdermal Drug Delivery Systems Market
https://www.marketresearchfuture.com/reports/transdermal-drug-delivery-systems-market-7545
EHR‑EMR Market
https://www.marketresearchfuture.com/reports/ehr-emr-market-819
Viscosupplementation Market
https://www.marketresearchfuture.com/reports/viscosupplementation-market-7255
Healthcare Predictive Analytics Market
https://www.marketresearchfuture.com/reports/healthcare‑predictive‑analytics-market-7549
Veterinary/Animal Vaccines Market
https://www.marketresearchfuture.com/reports/veterinary-animal-vaccines-market-2687
Electronic Skin Patches Market
https://www.marketresearchfuture.com/reports/electronic-skin-patches-market-7568
Medical Vacuum Systems Market
https://www.marketresearchfuture.com/reports/medical‑vacuum‑systems-market-7569
Anti-Aging Services Market
https://www.marketresearchfuture.com/reports/anti‑aging‑services-market-7579
About Market Research Future:
Market Research Future (MRFR) is a global market research company that takes pride in its services, offering a complete and accurate analysis with regard to diverse markets and consumers worldwide. Market Research Future has the distinguished objective of providing the optimal quality research and granular research to clients. Our market research studies by products, services, technologies, applications, end users, and market players for global, regional, and country level market segments, enable our clients to see more, know more, and do more, which help answer your most important questions.
Sagar Kadam
Market Research Future
+1 628-258-0071
email us here
Visit us on social media:
LinkedIn
Facebook
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.