Minimal Residual Disease Market to reach over USD 3.73 billion by the year 2030-InsightAce Analytic
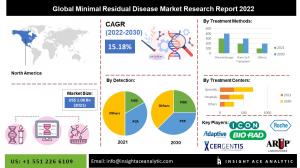
Global minimal residual disease market is estimated to reach over USD 3.73 billion by 2030, exhibiting a CAGR of 15.18% during the forecast period
NEW JERSEY, NJ, USA, November 21, 2022 /EINPresswire.com/ -- InsightAce Analytics Pvt. Ltd. announces the release of a market assessment report on the "Global Minimal Residual Disease Market (By Indication ( Leukemia, Acute Lymphoblastic Leukemia (All), Chronic Lymphoblastic Leukemia (CLL), Acute Myeloid Leukemia (AML, Chronic Myeloid Leukemia (CML), Others, Lymphoma, Non-Hodgkin's Lymphoma (NHL), Marginal Zone Lymphoma, Follicular Lymphoma, Peripheral T-Cell Lymphoma, Mantle Cell Lymphoma, Others, Multiple Myeloma (MM), Solid Tumor, And Others), Detection (Next-Generation Sequencing (NGS), Flow Cytometry, Polymerase Chain Reaction (PCR), Fluorescence In-Situ Hybridization (FISH) And Others), Treatment Method (Chemotherapy, Stem Cell Transplant, Cart Cell Therapy And Others) And Treatment Centres (Hospitals, Oncology Treatment Centers, Diagnostic Centers & Research Institutions, Speciality Clinics And Others))- Market Outlook And Industry Analysis 2030"
The global minimal residual disease market is estimated to reach over USD 3.73 billion by 2030, exhibiting a CAGR of 15.18% during the forecast period.
Request Sample: https://www.insightaceanalytic.com/request-sample/1448
The minimal residual illnesses are leukemic cells that remain in a patient during or after treatment. Cancer treatment, such as chemotherapy or radiotherapy, is not always effective in eradicating all carcinogenic cells and may also cause cancer. Organ transplants requiring a major histocompatibility test may be one of the instances where minimal residual disease testing is used for greater specificity. The highly financed oncology testing market is predicted to increase the number of technologies and the specificity of each test, hence assisting in the growth of the minimal residual disease industry. The increased prevalence of hematological malignancy and cancer and rising healthcare spending are expected to drive growth in the minimal residual disease sector throughout the forecast period. The increased government and private sector investment in MRD research and increased consumer awareness of personalized therapy are propelling market expansion. On the other hand, the high expense of research and development operations is limiting market expansion. Furthermore, the increase in R&D activities, the rise in tailored medications for treatment, and the spike in product development collaborations are all potential for market expansion. Additionally, rising demand for DNA testing due to developments in next-generation sequencing instruments and technologies, faster results, and cost-effectiveness compared to genotyping-based DNA microarray are driving market expansion.
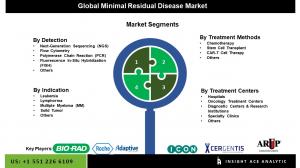
Prominent Players in the Minimal Residual Disease Market:
• Adaptive Biotechnologies
• ARUP Laboratories
• Bio-Rad Laboratories Inc.
• Cergentis B.V.
• F. Hoffmann-La Roche Ltd.
• ICON Plc
• Invivoscribe Inc.
• Laboratory Corporation of America Holdings
• Mission Bio
• Natera Inc.
• NeoGenomics Laboratories
• OPKO Health Inc.
• Sysmex Corporation
Market Dynamics:
Drivers-
The rising number of cancer patients and increased R&D spending are driving the expansion of the minimal residual disease market. Furthermore, the market is growing because of the increased implementation of next-generation sequencing (NGS) technology. Furthermore, technical developments in diagnostics and therapeutics due to increased R&D expenditure are boosting market expansion. Besides, favourable government and private-sector initiatives for developing and adopting NGS technologies, technological advancements in cloud computing and the availability of a technologically advanced healthcare research framework are all stimulating market growth. In addition, the better regulatory and reimbursement landscape for NGS-based diagnostic tests and expanded genome mapping activities will drive market expansion throughout the projection period.
Challenges:
The high expense of research and development operations is limiting market expansion. The healthcare, industrial, technology, and pharmaceutical industries often incur the highest R&D costs. Furthermore, despite significant progress toward expanding next-generation sequencing applications from research to the clinic, next-generation sequencing still presents massive challenges in quality control management, processing, data storage, and interpretation, slowing the translation from benchtop to bedside. This aspect is also impeding market expansion.
Regional Trends:
The North American minimal residual disease market is estimated to register a major share in the market during the forecast period. Because of rising healthcare costs in the United States, North America dominates the minimal residual disease industry. Furthermore, the rise in drug discovery platforms that need NGS technology and the increased emphasis of companies on creating treatments and diagnostics for detecting minimum residual disease are propelling market expansion in this region. Besides, Asia Pacific had a substantial share in the minimal residual disease market. The growing number of hospitals in emerging regions such as Asia Pacific will provide considerable potential opportunities for the minimal residual disease industry.
Enquiry Before Buying: https://www.insightaceanalytic.com/enquiry-before-buying/1448
Recent Developments:
• In October 2021-Inivata Limited Company, a subsidiary of NeoGenomics, Inc., engaged in a clinical partnership with the Princess Margaret Cancer Center in Toronto, Canada, to employ Inivata's InVisionFirst-Lung & RaDaR liquid biopsy assays in two separate studies.
• In May 2021-Invivoscribe company, for example, announced licensing of critical software and two new minimal residual disease clinical services. LymphoTrack Enterprise Software from the company helped high-volume customers manage escalating testing demands.
Segmentation of Minimal Residual Disease Market-
By Indication
• Leukemia
o Acute Lymphoblastic Leukemia (All)
o Chronic Lymphoblastic Leukemia (CLL)
o Acute Myeloid Leukemia (AML)
o Chronic Myeloid Leukemia (CML)
o Others
• Lymphorma
o Non-Hodgkin's Lymphorma (NHL)
o Marginal Zone Lymphorma
o Follicular Lymphorma
o Peripheral T-cell Lymphorma
o Mantle Cell Lymphorma
o Others
• Multiple Myeloma (MM)
• Soild Tumor
• Others
By Dectection
• Next-Generation Seqencing (NGS)
• Flow Cytomerty
• Polymerase Chain Reaction (PCR)
• Fluorescence In-Situ Hybridzation (FISH)
• Others
By Treatment Methods
• Chermotherapy
• Stem cell Transplant
• CAR-T Cell Therapy
• Others
ByTreatment Centers
• Hospitals
• Oncology Treatment Centers
• Diagnostic Centers & Research Institutions
• Specialty Clinics
• Others
By Region-
North America-
• The US
• Canada
• Mexico
Europe-
• Germany
• The UK
• France
• Italy
• Spain
• Rest of Europe
Asia-Pacific-
• China
• Japan
• India
• South Korea
• South East Asia
• Rest of Asia Pacific
Latin America-
• Brazil
• Argentina
• Rest of Latin America
Middle East & Africa-
• GCC Countries
• South Africa
• Rest of Middle East and Africa
For Customization: https://www.insightaceanalytic.com/customisation/1448
Priyanka Tilekar
Insightace Analytic Pvt. Ltd.
+1 551-226-6109
email us here