Lenoss Medical to present cementless, physiological spinal fracture treatment solution at SIR 2022
The OsteoPearl Cementless VBA System augments fractured osteoporotic spines with new bone, supporting the natural healing process with simple, holistic solution
Physicians will now be able to choose a biological alternative which will enable them to eliminate the current challenges and risks associated with the use of liquid cements.”
PROVIDENCE, RI, USA, June 10, 2022 /EINPresswire.com/ -- Pioneering physiological treatment for osteoporotic vertebral compression fractures, Lenoss Medical is excited to announce its inaugural attendance at SIR 2022 - the Society of Interventional Radiology’s annual meeting in Boston - where it will showcase for the first time its OsteoPearl Cementless VBA System.— Dom Messerli, Lenoss Medical President & CEO
The numbers are staggering. Worldwide, 1 in 3 women and 1 in 5 men over the age of 50 will experience a painful and potentially debilitating osteoporotic fracture1. In the United States, an estimated 1.5 million vertebral compression fractures occur each year. Underdiagnosis of vertebral fracture is a worldwide problem. It is estimated that 45% go unrecognized during local assessment of patient radiographs (1). For patients undergoing proper surgical treatment, studies have shown a decrease in morbidity and mortality (2).
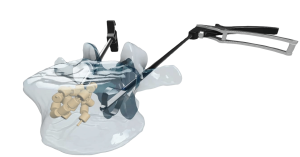
The OsteoPearl implant is the first fully natural biological, cementless technology for physiological fracture repair. The OsteoPearls consist of 100% cortical bone with well-established osteoconductive and -inductive osteointegration properties. By implantation of the OsteoPearl implants, the weak, osteoporotic spinal segment is augmented with allograft bone, allowing for the natural induction of new bone formation for the physiological healing of the fracture (3).
“We are excited to present a cementless, physiological fracture treatment solution for patients suffering from osteoporotic spinal fractures,” said Lenoss Medical’s president and CEO, Dom Messerli. “By eliminating the need for traditional, liquid acrylic cement and utilizing the natural properties of bone, we believe we will elevate the standard of care to the next level by allowing the fractures to heal physiologically. Physicians will now be able to choose a biological alternative which will enable them to eliminate the current challenges and risks associated with the use of liquid cements.”
Risk Reduction
Currently, the most common surgical treatments for vertebral compression fractures are cement vertebroplasty and balloon/cement kyphoplasty, both of which involve the injection of liquid acrylic cement into the fractured vertebra. Physicians take significant precautions to avoid unintended cement leakage outside the fracture, into the spinal canal, veins, and arteries, potentially causing pulmonary or intracardiac embolism (4,5,6). The OsteoPearl implants are made of non-liquid solid bone, use zero cement, leave behind no foreign bodies, and release no vapors, while augmenting the fracture with 100% cortical bone, with high compressive strength along with osteoconductive and osteoinductive properties.
What Makes the OsteoPearls Different
OsteoPearl implants are a simple biological solution. Made from 100% natural allograft tissue, they consist of flexible links connecting the cortical pearls. Delivered via the standard, minimally invasive surgical approach, the implants create a three-dimensional cortical structural matrix. By introducing both osteoconductive and osteoinductive properties, the patient’s natural healing process starts, and bone induction can begin (3). This approach is designed with the long-term health of the patient in mind, avoiding cardiac, pulmonary, and neurologic complications associated with traditional cement (4,5,6).
“We created the OsteoPearl with the belief that treatment for vertebral fractures should be natural and work in harmony with the patient's physiology,” Messerli said. “Leveraging several decades of allograft tissue use in orthopedics, we believe adding the OsteoPearl bone implants to where bone is needed makes the most sense, especially for patients with compromised bone health. OsteoPearl acts like bone because it is bone. Supporting the physicians to eliminate the risk of intracardiac and pulmonary cement embolism will benefit the patients by not compromising their heart and lung health while promoting their bone health.”
Medical Innovation
With a focus on simplifying the OR experience, the OsteoPearl Cementless VBA System consists of the OsteoPearl implants, access instruments, and the patented Elevoss cavity creator, thoughtfully designed to streamline pre-operative setup and minimize back table footprint and associated medical waste.
Lenoss Medical will be on display from June 12 through 14 during its inaugural attendance at this year’s Society of Interventional Radiology Annual Scientific Meeting in Boston. The product solution will be shown at Booth 343 and during the hands-on workshop “HOW: Advanced Interventional Pain Management” on Tuesday, June 14 from 3 p.m. to 4:30 p.m.
About Lenoss
Lenoss Medical, a Rhode Island-based medical device company, consists of proven industry professionals supported by a trusted advisory group of clinical experts all with a common goal of advancing our mission toward a physiological fracture treatment pathway.
1. International Osteoporosis Foundation. Epidemiology of osteoporosis and fragility fractures. www.osteoporosis.foundation/facts-statistics/epidemiology-of-osteoporosis-and-fragility-fractures
2. Morbidity and Mortality after Vertebral Fractures: Comparison of Vertebral Augmentation and Nonoperative Management in the Medicare Population. SPINE Aug 2015, Edition AA et al.; Survival and Cost Comparison of Kyphoplasty and Percutaneous Vertebroplasty Using German Claims Data. SPINE Feb. 2014, Lange A. et al.; Were VCF patients at higher risk of mortality following the 2009 publication of the vertebroplasty “sham” trials? Osteoporosis Int. Feb. 2018, Ong KL, Beall D., et al
3. Urist MR, Peltier LF. Bone: Formation by Autoinduction, Clinical Orthopaedics and Related Research. 2002:395 - Issue - p 4-10
4. Gabe Weininger, B.S., and John A. Elefteriades, M.D., Ph.D. Intracardiac Cement Embolism, October 2, 2021, NEJM.org
5. Cement_pulmonary embolism_after_percutaneous_kyphoplasty:_An unusual culprit for non–thrombotic_pulmonary embolism; Zalak Patel, MD, Rahul Sangani, MD b , Cara Lombard, MD Department of Radiology, West Virginia University, Morgantown, WV Department of Pulmonary and Critical Care Medicine, West Virginia University, Morgantown, WV
6. Leakage of cement in percutaneous transpedicular vertebroplasty for painful osteoporotic compression fractures; J. S. Yeom, W. J. Kim, W. S. Choy, C. K. Lee, B. S. Chang, J. W. Kang; From Eulji University Hospital, Daejon, Korea
Nancy Krause
Practice Marketing & Communications
nancy@practicemarcomm.com
Introducing OsteoPearl VBS System