Global warming has been much greater in urban areas than in rural areas
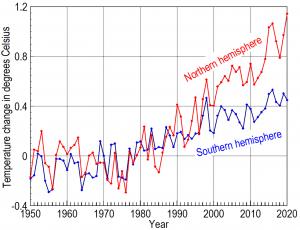
The northern hemisphere, which contains 90% of world population, most major cities, and most air pollution, warmed 70% more than the southern hemisphere
Ozone depletion played a major role in global warming since 1970 and can explain quite directly why urban areas are warmer than rural areas.”
JACKSON, WYOMING, UNITED STATES, December 10, 2020 /EINPresswire.com/ -- All major analyses of average global temperatures showed temperatures remained relatively constant from 1950 through 1970. Average temperatures from 2014 through 2019, however, increased 1.7℉ in the northern hemisphere but only 1.0℉ in the southern hemisphere.— Dr. Peter L. Ward, U.S. Geological Survey retired
Meteorologists have noted for decades that air temperatures in urban areas tend to be substantially warmer than air temperatures in surrounding suburban areas. This so-called urban heat island effect is thought to be caused primarily by human modification of land surfaces. Dark roads and most building surfaces absorb more solar radiation than trees and other vegetation. Trees also provide more shade and more evaporative cooling.
“But there is another process active in urban areas, the dissociation of ozone which has been widely ignored”, says Dr. Peter L. Ward, a geophysicist who worked 27 years with the U. S. Geological Survey. Ward is presenting his conclusions at the annual meeting of the American Geophysical Union (AGU) on December 15. AGU is the largest organization in the world for scientists studying Earth and space systems with 60,000 members from 135 countries. This year’s meeting includes more than 20,000 scientists contributing virtually.
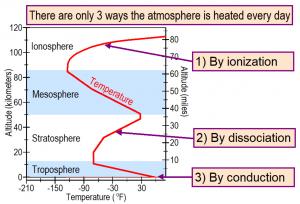
Ward explains in his presentation that there are only three known ways that solar radiation is observed every day to heat Earth’s atmosphere. The first is by conduction when air touches Earth’s sun-warmed surface and the warmed air rises. This is how air is heated above a hot frying pan. And this is how the troposphere, the lowest layer in Earth’s atmosphere, where we live, is heated from below when sunlight is available.
Temperature in the troposphere decreases with increasing altitude up to the tropopause, the boundary between the troposphere heated from below by conduction and the stratosphere heated from above by solar radiation. The tropopause is normally at an altitude close to 11 miles above the tropics, but only 5.6 miles above polar regions with temperatures typically 130℉ cooler than Earth’s surface. Airplanes normally fly near the top of the troposphere.
The second way air is heated is by dissociation. Dissociation primarily of molecular oxygen causes the top of the stratosphere, at altitudes of around 32 miles, to be 106℉ warmer than the tropopause at the base of the stratosphere. When a molecule of oxygen, containing two atoms of oxygen absorbs solar ultraviolet-C radiation, the molecular bond is dissociated, which means broken apart. The two oxygen atoms fly apart at high velocity just like the ends of a snapped rubber band. Air temperature is proportional to the average velocity of all atoms and molecules in the air squared. In this way, dissociation turns chemical bond energy directly and efficiently into air temperature.
Then, two atoms of oxygen can collide without reducing air temperature and be dissociated again, making the air warmer and warmer in a cycle that continues until all solar ultraviolet-C radiation has been absorbed before it reaches the base of the stratosphere.
Similarly, a molecule of ozone, containing three oxygen atoms, is dissociated when it absorbs solar ultraviolet-B radiation. The atom of oxygen and the molecule of oxygen fly apart at high velocity, warming the ozone layer. They can recombine by collision and then be dissociated again in a cycle that continues until all solar ultraviolet-B radiation has been absorbed.
Normally, nearly all solar ultraviolet-B radiation is absorbed in the ozone layer. But when the ozone layer is depleted by some chemical process, less ultraviolet radiation is absorbed in the ozone layer, cooling the ozone layer. More ultraviolet-B radiation is observed to reach Earth where it dissociates ground level ozone pollution, warming polluted air in urban and industrial areas.
The third way air is heated is by ionization that only occurs in the ionosphere, located above the stratosphere. Very high-energy solar radiation separates electrons from atoms and molecules. The pieces fly apart at high velocity, warming the ionosphere.
Ozone is the main chemical ingredient of smog in urban areas. Ground-level ozone pollution is created by chemical reactions between oxides of nitrogen and volatile organic compounds. These reactions are common when pollutants emitted by cars, power plants, industrial boilers, refineries, chemical plants, and other sources react chemically in the presence of sunlight. Ozone pollution damages lungs, irritates throats causing coughing and shortness of breath, and can cause chest pain.
“It turns out,” Ward explains in his presentation, “that no physical way has even been demonstrated for air to be warmed when greenhouse gases absorb infrared radiation from Earth, which does not have enough energy to dissociate or ionize anything.”
The ozone layer is especially important for life on Earth because it normally absorbs most ultraviolet-B radiation that has enough energy to cause sunburn, skin cancer, cataracts, and mutations. Since 1970, you have been at higher risk for these problems.
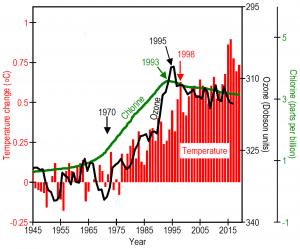
In the 1960s, manufactured chlorofluorocarbon gases (CFCs) became widely used as spray-can propellants, refrigerants, solvents, and foam blowing agents. Ozone depletion and average global surface temperatures began increasing. In 1974, scientists discovered that CFCs can be broken down by ultraviolet radiation in the stratosphere, freeing atoms of chlorine, and that one atom of chlorine, under the right conditions, can destroy 100,000 molecules of ozone. This work earned the Nobel Prize in Chemistry in 1995.
The United Nations passed the Montreal Protocol on Substances that Deplete the Ozone Layer, severely restricting production of CFCs beginning in 1989. By 1993 the increase in atmospheric concentrations of CFCs stopped. By 1995, the increase in ozone depletion stopped. By 1998, the increase in global temperatures stopped. Humans caused the world to warm 1.1℉ from 1975 to 1998. Humans then stopped the warming by passing the Montreal Protocol.
“Without the Montreal Protocol,” Ward explains, “Earth would probably be at least 0.5℃ warmer today. CFCs are very stable substances. Unfortunately, it will take decades for the ozone layer to recover. Meanwhile temperatures will remain warm in urban areas.”
Ward concludes “ozone depletion played a major role in global warming since 1970 and can explain quite directly why urban areas are warmer than rural areas.”
More information as WhyClimateChanges.com
Peter Ward
Science Is Never Settled Inc.
+1 307-413-4055
email us here
Visit us on social media:
Facebook
Twitter
LinkedIn
Ozone depletion explains global warming better than greenhouse gases. A talk to the American Meteorological Society January 12, 2016.