Calimex USA Develops 7-Day Platelet, BacT Alert & Notification Application for South Texas Blood & Tissue Center
Paperless and Automated 7 Day Platelet Middleware by Calimex USA
SAN FRANCISCO, CALIFORNIA, USA, November 23, 2020 /EINPresswire.com/ -- Calimex USA successfully developed a new middleware called “BacT Alert & Notification Application” for South Texas Blood & Tissue Center (STBTC). The new BacT Alert & Notification Application connects bioMerieux’s new device, Virtuo and 3D via bioMerieux’s MYLA® data manager to STBTC’s Blood Enterprise Computer System (BECS) called ElDorado Donor®. STBTC will go live next month with our new BacT Alert & Notification Application that conforms to FDA MDDS requirements and is compliant to FDA Guidelines issued Sept. 2019.
We thank and appreciate BioBridge Global and STBTC for this opportunity.
Background
Calimex USA was awarded two projects by South Texas Blood & Tissue Center for the organization’s move from the current 5-day platelet process to the new 7-day process compliant to FDA Guidance for Industry, issued Sept. 2019.
1. Phase I Requirement Gathering – awarded in March 2020
2. Phase II Software Application Development and Interface Implementation – awarded in Aug. 2020
Phase I - Requirement Gathering
This involved a detailed business analysis of the current 5-day platelet process and interviewing all the stakeholders to arrive at an effective and efficient future state compliant to the new FDA Guidance for Industry, issued Sept. 2019. STBTC and BioBridge Global formed a strong project team to support Calimex. STBTC and BioBridge Global leadership was excellent in facilitating, collaborating, and working jointly with Calimex, resulting in a final Business Requirement Document (BRD). Sixty-two requirements were identified and documented in the BRD. The final BRD was reviewed and approved by BioBridge Global and STBTC management.
Phase II - BacT Alert & Notification Application Development -
Calimex developed a new BacT Alert & Notification Application conforming to FDA MDDS requirements and to all 62-and-more user requirements listed in the Business Requirement Document. Calimex BacT Alert & Notification Application includes BacT Testing Requisition Automation with an electronic manifest to take advantage of the new bioMerieux Device for increased efficiency. It takes advantage of bioMerieux’s MYLA® for an automated paperless process with alerts that includes Emails, Phone, SMS, Fax and built-in escalation processes. Detailed end-to-end (E2E) Testing is currently being performed.
** Note **
The BacT Alert & Notification Application is now available for other blood centers, plasma centers, labs and hospitals. Please email us - sam@calimex.net.
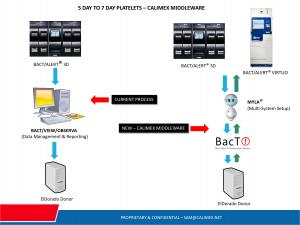
Process Architecture - See Image included:
About BioBridge Global
BioBridge Global (BBG) is a San Antonio, Texas-based 501(c)(3) nonprofit corporation that offers diverse services through its subsidiaries – the South Texas Blood & Tissue Center, QualTex Laboratories, GenCure and The Blood & Tissue Center Foundation. BBG provides products and services in blood resource management, cellular therapy, donated umbilical cord blood and human tissue, as well as testing of blood, plasma and tissue products for clients in the United States and worldwide. BBG is committed to saving and enhancing lives through the healing power of human cells and tissue. It enables advances in the field of regenerative medicine by providing access to human cells and tissue, testing services and biomanufacturing and clinical trials support. Learn more at BioBridgeGlobal.org.
About the South Texas Blood & Tissue Center
The South Texas Blood & Tissue Center (STBTC) is a nonprofit community blood center that provides blood, plasma, platelets and other blood components to 100 hospitals in 48 South Texas counties. It is the largest blood supplier in the region. In addition, it recovers and distributes donated human tissue for transplant. STBTC has a 45-year history serving the South Texas community. STBTC has seven donor rooms in South Texas and conducts hundreds of mobile blood drives each year. STBTC is online at SouthTexasBlood.org.
About Calimex USA
Calimex USA Corporation is an American information technology company specializing in the regulated healthcare environment. It is a leading provider of specialized healthcare solutions with over three decades of experience in developing and delivering innovative solutions designed to ensure the highest levels of safety, security, efficacy, effectiveness and efficiency. Calimex takes complex clinical - and often painfully inefficient processes and automates them for positive return on investments (ROI).
www.calimex.net
Contacts:
Sam Waran CPA, MBA, CISM, CGEIT, CRISC, MCSE, OCP
Calimex USA Corporation
1 415 221 5515
Sam@Calimex.Net – Preferred Communication.
Sam Waran
Calimex USA Corporation
+1 415-505-9961
sam@calimex.net
Visit us on social media:
Facebook
Twitter
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.