CLINUVEL to Trial Innovative Drug in Stroke
MELBOURNE, Australia, Oct. 28, 2020 (GLOBE NEWSWIRE) -- The drug afamelanotide will be used for the first time in patients with acute stroke. The study will evaluate the safety and efficacy of afamelanotide, developed by Australian company CLINUVEL, in arterial ischaemic stroke (AIS). The aim is to offer a treatment for patients suffering a stroke who are unable to receive treatment to dissolve or remove the underlying blood clot. AIS accounts for approximately 85% of the 15 million strokes suffered worldwide each year.
“Stroke is most commonly caused by a clot in a patient’s brain which starves surrounding tissue of blood and essential oxygen, causing the destruction of brain cells,” CLINUVEL’s Chief Scientific Officer, Dr Dennis Wright said. “This brain damage can have an irreversible effect on a patient’s ability to speak, move, and function, and tragically leads to an early death for more than 5.5 million people per annum. It is our aim to show that treatment with afamelanotide can safely reduce and prevent brain damage in the majority of stroke patients who cannot be offered standard therapy.”
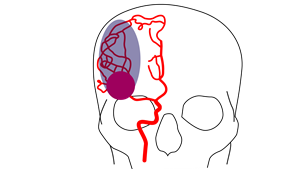
Strokes cause death of brain tissue at the site of the clot and lead to a shortage of oxygen in a larger area of the brain, known as the penumbra, which is salvageable brain tissue if treated quickly. The longer the delay in a stroke patient receiving treatment, the greater the potential threat to their life and overall prognosis as tissue within the penumbra becomes irreversibly damaged.
Current stroke therapies rely on early intervention to restore blood flow to the brain by either chemically dissolving or physically removing the clot. In Europe, no treatment can be offered to over 85% AIS patients due to a critical delay between the start of the stroke and presentation of the patient to a hospital. Additionally, the location of the clot within the artery is also an important factor impacting the possibility to offer treatment.
Research has indicated that afamelanotide – which is approved in Europe and the USA for patients with a rare metabolic disorder called EPP1 – may rapidly exert its effects to protect brain tissue, act on blood vessels to optimise blood flow, and reduce the size of swelling in the brain following a stroke. More than 10,000 doses of afamelanotide have been administered to over 1,400 individuals during its development and use across a period of nearly two decades.
The pilot Phase IIa clinical study (CUV801) will be conducted at a single expert neurological emergency centre, assessing the safety and effectiveness of an injectable controlled-release implant formulation of afamelanotide (SCENESSE®) in AIS patients. Six adult patients with clots located in the higher segments of the brain and who are ineligible for alternative treatments will be enrolled in the study and evaluated for six weeks.
CUV801 will assess patients’ brain injury with computed tomography (CT) scans and magnetic resonance imaging (MRI), as well as using recognised methods of clinical evaluation to measure changes in patients’ neurological and cognitive function following treatment.
“The objective of intervention with afamelanotide is to safely assist the restoration of blood flow and oxygen supply to the brain while minimising the traumatic damage and fluid accumulation. For our team, the ultimate aim is to reduce the overall damage stroke does to those patients,” Dr Wright said.
“Having monitored the real-world use of SCENESSE® in patients in Europe and the USA, we have now collected sufficient safety data to further our clinical programs in life threatening disorders. We look forward to the first study results in the first half of 2021, but also depending on the capacity of hospitals due to the COVID pandemic,” Dr Wright said.
Media enquiries
Monsoon Communications
Mr Rudi Michelson, 61 411 402 737, rudim@monsoon.com.au
Notes to editors:
A longer technical release has been issued to the Australian Securities Exchange and is available on CLINUVEL’s website www.clinuvel.com.
1 SCENESSE® (afamelanotide 16mg) is approved in the European Union and Australia as an orphan medicinal product and the world’s first systemic photoprotective pharmaceutical for the prevention of phototoxicity in adult patients with erythropoietic protoporphyria (EPP). SCENESSE® is approved in the USA to increase “pain- free” light exposure in adult EPP patients with a history of phototoxicity. Information on the product can be found on CLINUVEL’s website at www.clinuvel.com.
Authorised for ASX release by the Board of Directors of CLINUVEL PHARMACEUTICALS LTD
About CLINUVEL PHARMACEUTICALS LIMITED
CLINUVEL PHARMACEUTICALS LTD (ASX: CUV; NASDAQ INTERNATIONAL DESIGNATION ADR: CLVLY; XETRA-DAX: UR9) is a global and diversified biopharmaceutical company focused on developing and commercialising treatments for patients with genetic, metabolic, and life-threatening disorders, as well as healthcare solutions for the general population. As pioneers in photomedicine and understanding the interaction of light and human biology, CLINUVEL’s research and development has led to innovative treatments for patient populations with a clinical need for systemic photoprotection, DNA repair and acute or life-threatening conditions. These patient groups range in size from 5,000 to 45 million worldwide. CLINUVEL’s lead compound, SCENESSE® (afamelanotide 16mg), was approved by the European Commission in 2014 and the US Food and Drug Administration in 2019 for the prevention of phototoxicity (anaphylactoid reactions and burns) in adult patients with erythropoietic protoporphyria (EPP). More information on EPP can be found at http://www.epp.care. Headquartered in Melbourne, Australia, CLINUVEL has operations in Europe, Singapore and the USA. For more information please go to http://www.clinuvel.com.
SCENESSE® and PRÉNUMBRA® are two of several registered trademarks of CLINUVEL PHARMACEUTICALS LTD.
Head of Investor Relations
Mr Malcolm Bull, CLINUVEL PHARMACEUTICALS LTD
Investor enquiries
https://www.clinuvel.com/investors/contact-us
Forward-Looking Statements
This release contains forward-looking statements, which reflect the current beliefs and expectations of CLINUVEL’s management. Please see the CLINUVEL website for the full disclaimer – www.clinuvel.com.
A photo accompanying this announcement is available at: https://www.globenewswire.com/NewsRoom/AttachmentNg/fcd23834-07a7-4914-91b7-12969f6472b7
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.