A New Advancement in Preventing Tumor Lysis Syndrome (TLS) for Cancer Treatments Could Help Extend and Save Many Lives
SAINT PAUL, MN, USA, October 16, 2020 /EINPresswire.com/ -- Humanity Life Extension LLC, a Minnesota based company, announces it has developed a new and First of its Kind, patented technology that can prevent Tumor Lysis Syndrome (TLS), during fast cancer treatments that destroy cancer in just a few short hours.
Given that this is October and Breast Cancer Awareness Month, this new advancement gives hundreds of thousands of women hope for a viable Breast Cancer Treatment for even the most difficult, Triple Negative Breast Cancer, that in the past has not existed.
Many oncologists and doctors have known how to destroy cancers very quickly. The challenge was that the patient would die from TLS due to the destroyed highly acidic dead cancer matter and the patient's PH level dropping due to the acidic toxins. This overload of toxins and inflammatory mediators overwhelm the kidneys and liver to the point of failure. This new technology also prevents the horrible side effects of long drawn out chemotherapy treatments, which is the standard of care today.
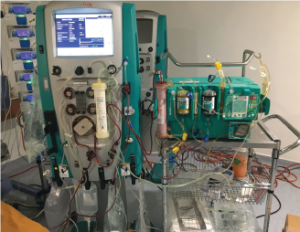
MN based Humanity Life Extension LLC has developed a method that can maintain a patient's normal PH level between 7.35 and 7.45 while at the same time maintaining their body in a perfect state of Physiological Homeostasis. They call their System PhyHom™, which seems fitting due to its function. The patient must be hooked up to the apparatus for it to work.
According to Researcher and CEO, Patrick Spearman, destroying cancer quickly is not all that difficult, however keeping the patient alive has been the challenge. With our PhyHom™ Toxin Removal System, tumor degradation or cellular breakdown products, along with free radicals, and inflammatory mediators will be removed at any rate created by the destroyed cancer.
The system employs a method of CRRT (continuous renal replacement therapy) in CVVH mode, which is a convection mode and not a diffusion method. The molecular size of the membrane in the CRRT equipment is large enough (about 60,000 daltons) to remove acids that in the past were believed to be unable to remove. Conventional kidney dialysis machines will not work according to Spearman. In addition, a method of albumin diffusion is employed to remove and protect the liver from any other toxins bound up in the patient's albumin in their blood stream that the CRRT machines do not eliminate. Also, the apparatus will eliminate excessive, and even prevent, edema.
If our PhyHom™ technology is combined with a method for destroying breast cancer in just a few short hours as an example, the patient will not succumb to TLS even at a stage 4 level with as much as 99% or more of the cancer being destroyed, according to Shelly Amann, the President of the company.
A renowned Head & Neck and Surgical Oncologist in New York, who is one of 40 board members on the Noble Prize committee, wrote to the company and called the entire Treatment a "potentially groundbreaking technology" and offered to assist Humanity Life Extension in any way that he could.
PhyHom™ technology will also be able to save countless lives on patients in intensive care for such ailments as pancreatitis or any other affliction that may cause a patient’s PH level to drop that would otherwise cause certain death.
Humanity Life Extension has performed GLP animal studies in the USA in an approved FDA laboratory to prove safety and then moved forward and performed their procedure in human trials in another country. The technology is ready for USA, FDA clinical safety trials, according to the company. The company is applying for an IDE from the FDA so they may begin trials in the United States along with their Humilty® Cancer Treatment which employs their PhyHom™ toxin removal system. Treatments for patient's are not available in the United States until USA clinical safety trials have been approved by the FDA. Investors or Institutions are welcome to contact the company for detailed information on both the technology and the investment.
Contact Information is:
Patrick Spearman - pspearman2@gmail.com 651-315-4583
Shelly Amann - samann@humanitylifeextension.com 651-315-4588
Patrick Spearman
Humanity Life Extension LLC
+1 651-315-4583
email us here